
In order to promote public education and public safety, equal justice for all, a better informed citizenry, the rule of law, world trade and world peace, this legal document is hereby made available on a noncommercial basis, as it is the right of all humans to know and speak the laws that govern them.

This Joint Australian/New Zealand Standard was prepared by Joint Technical Committee CH-026, Safety in Laboratories. It was approved on behalf of the Council of Standards Australia on 13 May 2010 and on behalf of the Council of Standards New Zealand on 27 August 2010. This Standard was published on 17 September 2010.
The following are represented on Committee CH-026:
Australian Industry Group
Australian Institute of Occupational Hygienists
CSIRO
Department of Labour, New Zealand
Department of Primary Industries, Vic.
Environmental Science and Research, New Zealand
Ministry of Agriculture and Forestry, New Zealand
Ministry of Economic Development, New Zealand
National Association of Testing Authorities, Australia
National Measurement Institute, Australia
New Zealand Chemical Industry Council
New Zealand Microbiological Society
RMIT University
Royal Australian Chemical Institute
WorkSafe Victoria
WorkCover New South Wales
Additional Interests:
Australasian Plant Pathology Society
Australian National University
Australian Quarantine and Inspection Service
Australian Society for Microbiology
Biosafety Consultant
Containment consultants
CSIRO, Division of Livestock Industries
Microbiologists
Office of The Gene Technology Regulator
Sterilizing Research Advisory Council of Australia, Vic.
Victorian Infectious Diseases Reference Laboratory
Keeping Standards up-to-date
Standards are living documents which reflect progress in science, technology and systems. To maintain their currency, all Standards are periodically reviewed, and new editions are published. Between editions, amendments may be issued. Standards may also be withdrawn. It is important that readers assure themselves they are using a current Standard, which should include any amendments which may have been published since the Standard was purchased.
Detailed information about joint Australian/New Zealand Standards can be found by visiting the Standards Web Shop at www.saiglobal.com.au or Standards New Zealand web site at www.standards.co.nz and looking up the relevant Standard in the on-line catalogue.
For more frequent listings or notification of revisions, amendments and withdrawals, Standards Australia and Standards New Zealand offer a number of update options. For information about these services, users should contact their respective national Standards organization.
We also welcome suggestions for improvement in our Standards, and especially encourage readers to notify us immediately of any apparent inaccuracies or ambiguities. Please address your comments to the Chief Executive of either Standards Australia or Standards New Zealand at the address shown on the back cover.
This Standard was issued in draft form for comment as DR 07335.
iiAS/NZS 2243.3:2010
Originated as AS 2243.3—1979.
Previous edition AS/NZS 2243.3:2002.
Sixth edition 2010.
COPYRIGHT
© Standards Australia Limited/Standards New Zealand
All rights are reserved. No part of this work may be reproduced or copied in any form or by any means, electronic or mechanical, including photocopying, without the written permission of the publisher, unless otherwise permitted under the Copyright Act 1968 (Australia) or the Copyright Act 1994 (New Zealand).
Jointly published by SAI Global Limited under licence from Standards Australia Limited, GPO Box 476, Sydney, NSW 2001 and by Standards New Zealand, Private Bag 2439, Wellington 6140
ISBN 978 0 7337 6996 2
1This Standard was prepared by the Joint Standards Australia/Standards New Zealand Committee CH-026, Safety in Laboratories, to supersede AS/NZS 2243.3:2002, Safety in laboratories, Part 3: Microbiological aspects and containment facilities.
Major changes in this edition are as follows:
The Committee is currently addressing the need to develop a section for containment of water based species, including fish and aquatic invertebrates. Some applicable information may be found in the laboratory and animal facility sections of this Standard.
The containment of plant pathogens is primarily concerned with minimizing hazards due to inadvertent spread to the environment. This is in contrast to the containment of human and animal pathogens, where the principal aim is to avoid risk of infection or contamination of facility workers and the community.
The containment of invertebrate pathogens may involve the minimization of hazards associated with inadvertent spread to the environment or microbiological hazards associated with exposure to people or animals. It may involve both of these hazards simultaneously. Where hazards to personnel are present in an invertebrate facility, the invertebrates and laboratory work will need to be carried out in a laboratory of appropriate microbiological containment level to protect the personnel, along with the additional containment features associated with invertebrate containment.
The Parts of the series promoting safety in laboratories are as follows:
| Part 1: | Planning and operational aspects |
| Part 2: | Chemical aspects |
| Part 3: | Microbiological safety and containment (this Part) |
| Part 4: | Ionizing radiations |
| Part 5: | Non-ionizing radiations—Electromagnetic, sound and ultrasound |
| Part 6: | Mechanical aspects |
| Part 7: | Electrical aspects |
| Part 8: | Fume cupboards |
| Part 9: | Recirculating fume cabinets |
| Part 10: | Storage of chemicals |
Although many of the safety aspects of working in laboratories are addressed in other Parts of the series, some are repeated here in Part 3 because there is an increase in the risk in containment facilities.
2This Standard is intended to cover safety and containment aspects of work with microorganisms, including genetically modified microorganisms. However, it does not cover the additional security requirement that may be implemented in response to community interest and concerns in genetic modification work. For these, the relevant regulatory authority should be consulted. Also, the Standard is not primarily intended to address containment of organisms for work that does not involve microorganisms.
This Standard is intended to assist in addressing the obligations placed on employers and employees under occupational health and safety legislation to take care of both themselves and others in the workplace. It should not be assumed that compliance with this Standard means that all aspects of appropriate legislation or all legal obligations are being fulfilled. This Standard is not intended to provide for compliance with a specific act or regulation.
It should be noted that nothing in this Standard is required by law in any jurisdiction unless the Standard has been specifically incorporated by an Act or regulation in that jurisdiction. The exact manner of incorporation will determine whether the whole document, or specific sections or provisions, are made legal requirements or whether the Standard becomes an Approved Code of Practice. However, it should also be noted that this Standard is recognized in common law as defining current knowledge in microbiological safety practice. The provisions in a Code are not mandatory but give practical guidance on how to comply with the relevant provisions of the Act or regulation. Provided an alternative method also fulfils the requirements of the Act or regulation, it may be used. Users will need to consult the relevant authority to determine if this Standard has been incorporated and the manner of incorporation, if any.
In recognition of the changes made to this Standard during its revision, existing facilities should be assessed for risk and interim control measures should be implemented.
Current facilities and procedures should be updated to conform to this Standard. Compliance improvements should be made within a time frame that takes into consideration the cost of upgrading and the severity of the associated risk.
The terms ‘normative’ and ‘informative’ have been used in this Standard to define the application of the appendix to which they apply. A ‘normative’ appendix is an integral part of a Standard and contains requirements that have to be met for compliance with the objectives and intent of this Standard. An ‘informative’ appendix is only for information and guidance.
3| Page | ||
| FOREWORD | 7 | |
| SECTION 1 SCOPE AND GENERAL | ||
| 1.1 | SCOPE | 8 |
| 1.2 | OBJECTIVE | 8 |
| 1.3 | REFERENCED DOCUMENTS | 8 |
| 1.4 | DEFINITIONS | 8 |
| 1.5 | ABBREVIATIONS | 12 |
| SECTION 2 ORGANIZATION AND RESPONSIBILITY | ||
| 2.1 | RESPONSIBILITY | 13 |
| 2.2 | QUARANTINE MATERIALS | 14 |
| 2.3 | LABORATORIES USING GENETICALLY MODIFIED ORGANISMS (GMOs) | 15 |
| 2.4 | LABORATORY BIOSECURITY | 16 |
| 2.5 | COMMISSIONING | 16 |
| 2.6 | HEALTH MANAGEMENT | 16 |
| 2.7 | INCIDENT REPORTING | 18 |
| 2.8 | EMERGENCY RESPONSE AND CONTINGENCY PLANS | 18 |
| SECTION 3 DEGREE OF HAZARD FROM MICROORGANISMS | ||
| 3.1 | GENERAL | 19 |
| 3.2 | CLASSIFICATION OF MICROORGANISMS BY RISK GROUP | 20 |
| 3.3 | RISK-GROUPING OF MICROORGANISMS BY TYPE | 22 |
| 3.4 | HUMAN AND ANIMAL CLINICAL AND DIAGNOSTIC SPECIMENS | 23 |
| 3.5 | QUALITY ASSURANCE OF CULTURES AND MATERIALS | 23 |
| 3.6 | WORK WITH HUMAN, ANIMAL OR PLANT CELLS | 24 |
| 3.7 | PRIONS | 24 |
| SECTION 4 PRINCIPLES OF CONTAINMENT | ||
| 4.1 | GENERAL | 35 |
| 4.2 | CONTAINMENT MEASURES | 35 |
| 4.3 | PHYSICAL CONTAINMENT CLASSIFICATIONS | 36 |
| 4.4 | LOCATION | 37 |
| SECTION 5 LABORATORY CONTAINMENT FACILITIES | ||
| 5.1 | LABORATORY PHYSICAL CONTAINMENT | 38 |
| 5.2 | REQUIREMENTS FOR PC1 LABORATORIES | 38 |
| 5.3 | REQUIREMENTS FOR PC2 LABORATORIES | 40 |
| 5.4 | REQUIREMENTS FOR PC3 LABORATORIES | 44 |
| 5.5 | REQUIREMENTS FOR PC4 LABORATORIES | 49 |
| SECTION 6 ANIMAL CONTAINMENT FACILITIES | ||
| 6.1 | REQUIREMENT FOR ANIMAL CONTAINMENT FACILITIES | 55 |
| 6.2 | PRINCIPLES OF ANIMAL CONTAINMENT | 55 |
| 6.3 | OTHER CONSIDERATIONS ASSOCIATED WITH ANIMAL CONTAINMENT | 56 |
| 6.4 | REQUIREMENTS FOR ANIMAL PC1 FACILITIES | 58 |
| 6.5 | REQUIREMENTS FOR ANIMAL PC2 FACILITIES | 60 |
| 6.6 | REQUIREMENTS FOR ANIMAL PC3 FACILITIES | 63 |
| 6.7 | REQUIREMENTS FOR ANIMAL PC4 FACILITIES | 69 4 |
| SECTION 7 PLANT CONTAINMENT FACILITIES | ||
| 7.1 | GENERAL | 75 |
| 7.2 | REQUIREMENTS FOR PLANT PC 1 FACILITIES | 75 |
| 7.3 | REQUIREMENTS FOR PLANT PC2 FACILITIES | 76 |
| 7.4 | REQUIREMENTS FOR PLANT PC3 FACILITIES | 79 |
| 7.5 | REQUIREMENTS FOR PLANT PC4 FACILITIES | 86 |
| SECTION 8 INVERTEBRATE CONTAINMENT FACILITIES | ||
| 8.1 | GENERAL | 90 |
| 8.2 | REQUIREMENTS FOR INVERTEBRATE PCI FACILITIES | 90 |
| 8.3 | REQUIREMENTS FOR INVERTEBRATE PC2 FACILITIES | 92 |
| 8.4 | REQUIREMENTS FOR INVERTEBRATE PC3 FACILITIES | 95 |
| 8.5 | REQUIREMENTS FOR INVERTEBRATE PC4 FACILITIES | 101 |
| SECTION 9 MICROBIOLOGICAL SPILLS | ||
| 9.1 | GENERAL | 106 |
| 9.2 | PLANNING | 106 |
| 9.3 | SPILLS INSIDE BIOLOGICAL SAFETY CABINETS | 107 |
| 9.4 | SPILLS OUTSIDE BIOLOGICAL SAFETY CABINETS | 108 |
| 9.5 | CENTRIFUGE SPILLS | 112 |
| SECTION 10 CHEMICALS, PPE AND SPECIAL EQUIPMENT | ||
| 10.1 | CHEMICALS | 114 |
| 10.2 | PERSONAL PROTECTIVE EQUIPMENT (PPE) | 114 |
| 10.3 | CENTRIFUGES | 116 |
| 10.4 | FREEZE-DRYING AND RECONSTITUTION OF CULTURES | 117 |
| 10.5 | LIQUID NITROGEN | 118 |
| 10.6 | PRESSURE STEAM STERILIZERS | 119 |
| 10.7 | BIOLOGICAL SAFETY CABINETS | 122 |
| 10.8 | LAMINAR FLOW CYTOTOXIC DRUG SAFETY CABINETS | 123 |
| 10.9 | HEPA FILTERS | 123 |
| SECTION 11 CLEANING | ||
| 11.1 | GENERAL | 125 |
| 11.2 | CLEANING PERSONNEL | 125 |
| 11.3 | CLEANING OF EQUIPMENT | 125 |
| 11.4 | WALLS AND SHELVES | 125 |
| 11.5 | FLOOR CLEANING | 125 |
| SECTION 12 CONTAMINATED MATERIALS AND WASTE | ||
| 12.1 | COLLECTION | 127 |
| 12.2 | DECONTAMINATION AND DISPOSAL OF WASTES | 127 |
| SECTION 13 TRANSPORT OF INFECTIOUS AND OTHER BIOLOGICAL MATERIALS | ||
| 13.1 | GENERAL | 130 |
| 13.2 | TRANSPORT REGULATIONS | 130 |
| 13.3 | TRANSPORT DEFINITIONS OF BIOLOGICAL MATERIALS | 131 |
| 13.4 | CLASSIFICATION AND PACKAGING | 131 |
| 13.5 | TRANSPORT OF INFECTED ANIMALS | 135 |
| 13.6 | DOCUMENTATION | 135 5 |
| APPENDICES | ||
| A | REFERENCED AND RELATED DOCUMENTS | 138 |
| B | EXAMPLE MICROBIOLOGICAL INCIDENT/ILLNESS REPORT FORM | 144 |
| C | ADDITIONAL CONTAINMENT REQUIREMENTS FOR POLIOVIRUS | 145 |
| D | BIOLOGICAL HAZARD SIGNS | 147 |
| E | WATER AND GAS SUPPLIES TO CONTAINMENT FACILITIES | 149 |
| F | CHEMICAL DISINFECTANTS | 153 |
| G | EXAMPLES OF RECOMMENDED LAYOUTS FOR PC3 AND PC4 FACILITIES | 164 |
| H | RECOMMENDATIONS ON ACCEPTABLE ROOM AIRTIGHTNESS | 169 |
Safety in all laboratories is primarily a management responsibility, but is also an individual responsibility. It is the responsibility of management to provide and maintain protective equipment and containment areas, a policy relating to safe work practices within a laboratory and to promote the training in, and institution of, those practices. It is the responsibility of the laboratory staff to carry out the safe work practices and to use protective equipment to minimize injury or prevent occupational illness, not only to themselves, but also to their colleagues. It is also a responsibility of managers to ensure that consideration is given to hazards to the general environment when dispensing or handling biological material. Staff training must be directed toward making safety an attitude of mind and an integral part of all laboratory procedures, so that a constant, purposeful control of the laboratory environment will result. Accidents such as spillages are an obvious hazard, but the production of aerosols during some routine procedures is a less obvious hazard that can be a serious source of contamination. In addition to the many problems commonly encountered in chemical laboratories, microbiological laboratories can pose the following specific problems:
The basic approach to working with microorganisms is to regard them as potential pathogens and to handle them with standard microbiological techniques. Nevertheless, microorganisms vary markedly in their pathogenicity. This Standard includes the classification of microorganisms into four risk groups and specifies work requirements for the corresponding four physical containment levels.
7STANDARDS AUSTRALAIA/STANDARDS NEW ZEALAND
Australian/New Zealand Standard
Safety in laboratories
Part 3: Microbiological safety and containment
This Standard sets out requirements, responsibilities and general guidelines relating to safe handling and containment of microorganisms and prions in laboratories. It includes animal, plant and invertebrate containment facilities (these may be integral or separate to the laboratory) where microbiological work such as research, teaching, diagnosis, quality control and regulatory analysis, e.g. of foodstuffs, water and effluents, pharmaceuticals and cosmetics, is undertaken. It may also provide assistance to other laboratories where specimens that may contain pathogenic microorganisms and prions are handled, e.g. biochemistry and soil laboratories. This Standard should be read in conjunction with AS/NZS 2243.1, AS/NZS 2982.1, building codes in Australia and New Zealand and other relevant Parts of the AS/NZS 2243 series.
NOTES:
The objective of this Standard is to provide management and staff of laboratories and containment facilities with requirements and guidelines that promote microbiological safety and prevent the unintended spread of microorganisms and prions.
Documents referred to in this Standard are listed in Appendix A.
For the purpose of this Standard, the definitions below apply.
Suspension in air of finely dispersed solids or liquids.
8NOTE: Any procedure that disrupts the surface of a liquid has potential to produce aerosols. Procedures such as shaking, mixing and ultrasonic disruption are particularly common examples for microbiological work.
A separate, fully-enclosable space with two doors designed to limit pressure fluctuations during entry and exit. A shower airlock is an airlock that incorporates full body shower capability, which can be used as part of exit procedures.
A separate, fully-enclosable space used during access and egress that has specific containment functions.
Substance capable of destroying or preventing growth of microorganisms under prescribed conditions of use, and specifically for application to living tissues.
The exercise of special procedures for maintaining—
The potential microbiological source of harm.
Cabinets intended to provide protection from hazardous biological agents for personnel and the environment. The cabinets are exhaust ventilated, with an inward flow of air away from the operator and high-efficiency particulate air (HEPA) filtration of exhaust air.
Cabinets intended to provide protection from hazardous biological agents for personnel and the environment and also to protect the material used in the cabinet from exogenous contamination. The cabinets provide this protection by inducing an inflow of air through the work access opening, by delivering recirculated, filtered, laminar flow air downwards through the work zone and by HEPA filtration of exhaust air.
Totally enclosed, ventilated cabinets that allow work to be performed through the use of attached gloves. These cabinets are gas-tight, maintained under a negative air pressure, have their supply air HEPA-filtered and have their exhaust air passed through two HEPA filters in series. Transfer boxes allow passage of materials into and out of the work zone while maintaining the negative pressure.
The containment principles, technologies and practices that are implemented to prevent the unintentional exposure to biological agents and toxins, or their accidental release.
A committee that provides advice, resources and facilities as are necessary for safe working in laboratories.
9NOTE: An institutional biosafety committee (IBC) is specific to gene technology. An institutional biological safety committee (IBSC) is the New Zealand equivalent of an IBC.
A separate, fully-enclosable space used by personnel for donning facility clothing and PPE on entry and for removing it on exit.
Space used by personnel to remove personal clothing as appropriate to facility level prior to entry and to don personal clothing on exit, e.g. from shower airlock.
A person who has acquired through training, qualifications or experience, or a combination of these, the knowledge and skills enabling that person to perform a specified task.
The combination of buildings, engineering function, equipment, and worker practices used to handle microorganisms and prions safely.
May comprise a combination of laboratories, animal, plant and invertebrate facilities and associated rooms within a physical containment barrier. This may include airlocks, access and support rooms and interconnecting corridors.
NOTE: This definition is distinct from that used in the MAF Biosecurity New Zealand and ERMA New Zealand Standard Facilities for Microorganisms and Cell Cultures: 2007a.
The undesirable transfer of microorganisms from one source to another.
A physical or chemical process that kills or removes pathogenic microorganisms, but does not necessarily result in sterility.
Any human, animal, plant or invertebrate material including, but not limited to, excreta, secreta, blood and its components, tissue and tissue fluids submitted for purposes of diagnosis or analysis.
A substance capable of killing a wide range of microorganisms; its use is usually confined to hard surfaces.
The airborne concentration of a particular substance (not microorganisms) in the worker’s breathing zone, exposure to which, according to current knowledge, should not cause adverse health effects nor cause undue discomfort to nearly all workers. The exposure standard can be of three forms; time-weighted average (TWA), peak limitation, or short term exposure limit (STEL).
A ‘high-efficiency particulate air’ (HEPA) filter complying with the requirements in Clause 10.9.1.
A microorganism capable of invading a susceptible host and multiplying in it, which may or may not cause a disease.
10Includes all multi-cellular animal species without backbones that are land based as adults. This includes annelids, cnidarians, echinoderms, flatworms, nematodes, molluscs and arthropods. Protozoans are not included due to their microscopic size and single cellular nature.
The potential microbiological source of harm, often called a ‘biohazard’.
An incident where control of infectious material has been lost inadvertently.
Microscopic organisms including protozoa and other parasites, fungi, archaea, bacteria, unicellular algae, viruses and viroids.
An infectious organism, usually microscopic, capable of causing disease in a host.
Any devices or equipment, including clothing, designed to be worn or held by a person on its own, or as part of a system, to protect against one or more health and safety hazards.
Proteinaceous infectious particles that lack nucleic acids, which can cause scrapie and other related neurodegenerative diseases of humans and animals.
A process of estimating the potential of a hazard (source of harm) to give rise to an adverse outcome. This estimation is based on a combination of the likelihood of the hazard occurring and the consequences if the hazard occurs. Control measures are used to limit the risk.
Indicates a statement is mandatory.
Objects or devices having sharp points or protuberances or cutting edges, capable of cutting or piercing the skin.
NOTE: See AS 4031 for information on sharps containers.
Indicates a recommendation.
The state of being free from viable microorganisms.
NOTE: In practice, no such absolute statement regarding the absence of microorganisms can be proven.
A validated process used to render a product free from viable microorganisms.
11NOTE: The number of microorganisms that survive a sterilization process can be expressed in terms of probability. While the probability may be reduced to a very low number, it can never be reduced to zero.
Living: capable of growth even though resuscitation procedures may be required, e.g. when microorganisms are sub-lethally damaged by being frozen, dried, heated or affected by chemicals and disinfectants.
For the purpose of this Standard, the abbreviations below apply:
| AQIS | Australian Quarantine and Inspection Service |
| BC | Biosafety committee |
| BSC | Biological safety cabinet |
| CWA | CEN Workshop Agreement |
| DNA | Deoxyribonucleic acid |
| ERMA | Environmental Risk Management Authority |
| GMO | Genetically modified organism |
| HEPA | High-efficiency particulate air |
| HSNO | Hazardous Substances and New Organisms |
| I AT A | International Air Transport Association |
| IBC | Institutional biosafety committee |
| IBSC | Institutional biological safety committee |
| IMO | International Maritime Organization |
| MAP BNZ | Ministry of Agriculture and Forestry Biosecurity New Zealand |
| NHMRC | National Health and Medical Research Council |
| NRL | National Radiation Laboratory |
| NT AC | National Tuberculosis Advisory Committee |
| OGTR | Office of Gene Technology Regulator |
| OHS | Occupational health and safety |
| PC | Physical containment |
| PPE | Personal protective equipment |
| RPE | Respiratory protective equipment |
Management shall provide staff with a policy statement on laboratory safety that recognizes the special hazards associated with microorganisms.
Research, teaching or operational work with biohazards shall only be undertaken after a risk assessment of the work has been conducted and it has been demonstrated that any hazards are controlled. This process shall be documented and regularly reviewed to ensure its ongoing validity. Review shall be undertaken whenever a change to the parameters of the original risk assessment is planned. See CWA 15793 and AS/NZS ISO 31000.
The risk assessment shall be done prior to commencement of any work to determine the appropriate type and level of containment facility. The risk assessment should include consideration of the following:
All risk assessments that involve biological systems are subject to a level of uncertainty due to a lack of experimental evidence. The level of uncertainty should be considered when conducting the risk assessment.
As an overall principle under occupational health and safety (OHS) laws, the employer is responsible for ensuring that the workplace is safe and free from risks to health.
13Organizational arrangements put in place to meet management responsibilities will depend on—
The central element of such arrangements is a Biosafety Committee (BC). The terms of reference of the BC in relation to biohazards should be as follows:
A safety officer shall be available to provide advice and guidance on microbiological safety.
The supervisor shall ensure that safe procedures are documented and put into practice. The supervisor shall implement initial and continuing training programs, ensure staff are supervised and ensure maintenance is carried out in accordance with safe procedures. The supervisor should also ensure that casual visitors do not have unrestricted access to the laboratory.
All laboratory work shall be carried out with regard to the safety of laboratory occupants. The following requirements apply to all laboratory personnel:
The Australian Quarantine and Inspection Service (AQIS) approves places where post-entry quarantine requirements apply for a wide range of human, plant and animal pathogens and human, plant and animal products, so that it can be sure that these activities are performed with a minimal degree of risk.
14To gain approval as a quarantine approved premises (QAP), there are conditions that the premises must meet to assure AQIS that the risk of any quarantine breach is minimal. These conditions detail the requirements and responsibilities for containment facilities where the premises are utilized for research, analysis or testing of imported material, including microorganisms, animal and human products and soil. Premises of this type include microbiological, animal, plant and invertebrate facilities.
Further information on the approval process for quarantine approved premises is available at http://www.daff.gov.au/aqis/import/general-info/qap.
AQIS will also require institutions to obtain a permit for work conducted in containment facilities. Such permits contain special conditions. The import conditions database (ICON) should be consulted at http://www.aqis.gov.au/icon32/asp/homecontent.asp.
In New Zealand, Ministry of Agriculture and Forestry Biosecurity New Zealand (MAF BNZ) controls the import of biological risk goods, establishes Standards for laboratories receiving and holding such goods and enforces the compliance to those standards.
For further information, contact—
Import Standards Group
MAF BNZ
Box 2526
Wellington New Zealand
Website: www.maf.govt.nz
Under the Commonwealth gene technology legislation, certain dealings with genetically modified organisms (GMOs) are required to be conducted in facilities that meet and are certified as complying with OGTR Guidelines for the certification of physical containment facilities.
Organizations seeking information about the regulatory requirements that apply to work with GMOs should contact the OGTR for further information at the following address:
Office of the Gene Technology Regulator
MDP 54
GPO Box 9848
CANBERRA ACT 2601
Website: www.ogtr.gov.au
Under the Hazardous Substances and New Organisms (HSNO) Act 1996, work with genetically modified organisms is regulated by the Environment Risk Management Authority (ERMA). In general, all work involving the holding or development of GMOs shall be carried out in accordance with controls set by ERMA, or an IBSC or the Chief Executive of ERMA New Zealand in containment facilities approved by MAF BNZ. Work involving GMOs shall be carried out in accordance with MAF BNZ and ERMA New Zealand Standard: Facilities for Microorganisms and Cell Cultures: 2007a.
15Laboratories should consult—
ERMA New Zealand
PO Box 131
Wellington
Email: enquiries@ermanz.govt.nz
Website: www.ermanz.govt.nz
Work involving GMOs shall be carried out in accordance with controls set by HSNO Act Approvals. Such work shall be carried out in MAF-BNZ-approved containment facility to the appropriate Standard.
Laboratory biosecurity, as opposed to laboratory biosafety, refers to the institutional and personal security measures designed to prevent the loss, theft, misuse, diversion or intentional release of pathogens and toxins. Facilities holding pathogens or toxins should prepare and implement a specific laboratory biosecurity program according to the requirements of the facility, the nature of the pathogens(s) or toxins(s), the type of laboratory work conducted and the local conditions. Measures should include a secure inventory of all microorganisms, including such information as location and access. All personnel in such facilities should be trained in biosecurity measures.
NOTE: In Australia, the regulation of security sensitive biological agents (SSBA) is governed by the National Health Security Act 2007 administered by the Commonwealth Department of Health and Ageing (www.health.gov.au/ssba).
On completion of the construction of a containment facility or major changes to a containment facility, the surrounding building, environment, associated air supply or exhaust systems, the facility shall be assessed for overall compliance with this Standard. An itemized checklist should be developed to assist in ensuring all requirements of the Standard relevant to the particular facility are taken into account when conducting the assessment.
All personnel shall be advised of the risk of occupational exposure to microorganisms to which they may not be immune.
When working with human pathogens of Risk Group 3 or Risk Group 4, each person working in the laboratory or animal, plant or invertebrate facility shall be subjected to an initial medical examination, including a chest X-ray where relevant, and periodic examinations. A baseline serum sample should be obtained from at risk personnel and stored for future reference. (See also Clauses 2.6.3 and 2.6.5.)
When working with human pathogens of Risk Group 4, a system shall be set up for reporting accidents and exposures to microorganisms, for monitoring employee absenteeism and for the medical surveillance of illnesses that are potentially laboratory associated.
16Minor cuts and abrasions, which provide routes for infection from contaminated surfaces, should be adequately covered and kept dry. Infections (especially respiratory or wound) can provide sources of contamination for experimental materials and fellow workers. Individual cases should be assessed in relation to the particular laboratory’s work. All injuries that occur in the workplace shall be reported to the supervisor (see Clause 2.7). Immediate medical action is required after human blood or body fluid exposure and contaminated sharps injuries. (See the Department of Health and Ageing publication. Infection control guidelines for the prevention of transmission of infectious diseases in the health care setting.) Consideration should be given to whether any infection was laboratory acquired.
A serum bank can be invaluable when there are questions of work-related infection. Subject to privacy and informed consent considerations, baseline serum samples should be collected from ‘at-risk’ personnel, to be stored for future reference. Additional serum samples may be collected periodically, depending on the risk of exposure to agents handled in the laboratory.
If samples are collected, procedures shall be documented defining who owns the serum, how it is stored, who can access it for testing, who may order tests, who evaluates the tests and who can have access to the results.
For those working with human or zoonotic pathogens, or samples that may contain human or zoonotic pathogens, the latest editions of The Australian Immunisation Handbook published by the NHMRC or the Immunisation Handbook published by the New Zealand Ministry of Health, as appropriate, should be consulted and implemented.
All new staff working with specimens and cultures potentially containing Mycobacterium tuberculosis (TB) complex are recommended to have a tuberculin skin test (TST) or TB gamma-interferon assay. Prior vaccination with Bacille Calmette-Guerin (BCG) confounds TST testing but not the TB gamma interferon assay (see Reference 1.1). Staff with negative test results should be retested on an annual basis (see the NTAC Guidelines for Australian Mycobacteriology Laboratories or Guidelines for Tuberculosis Control in New Zealand 2003, as appropriate).
Vaccination against M. tuberculosis is not generally recommended. However BCG vaccination should be considered for staff with high risk of exposure to TB and as recommended by State/Territory TB control authorities.
Consideration should be given to the immunization of support staff where appropriate.
Persons who are immuno-suppressed, immuno-compromised, or otherwise unduly vulnerable to infection, such as persons who are diabetic, should inform their supervisor or person responsible for microbiological safety of their condition so that appropriate action may be taken. Medical opinion may be required if working with human pathogens. Some microorganisms that are regarded as part of the normal flora of humans or animals may be pathogenic for immuno-compromised persons.
Laboratory management shall inform all female employees of the risk to the unborn child or the pregnant woman of occupational exposure to certain microorganisms (e.g. Toxoplasma gondii, Listeria monocytogenes, cytomegalovirus, parvovirus B19, rubella virus, human immunodeficiency virus (HIV), Coxiella burnetii and hepatitis B, C and E viruses) and some fungi. The precise steps taken for protection will vary, depending on the microorganisms to which the woman may be exposed. Medical opinion may be required.
17Where an incident has occurred resulting in an injury or illness, priority shall be given to the care of the injured or ill person. The project risk assessment should be consulted and consideration should be given to the Risk Group if a microorganism is involved, how it might be transmitted and if any hosts, the environment or processes are at risk as a result of the accident. First aid should be applied by trained personnel, ensuring that they do not risk being infected. If necessary, medical aid should then be sought (see also Clause 2.6.2). The incident should be reported verbally to the supervisor as soon as reasonably possible, documented using the institution’s health and safety report form and referred to the BC or referred as required by the equivalent institutional procedures.
Where an incident occurs with potential for contamination from infectious material, it should be reported verbally to the supervisor as soon as reasonably possible. The incident should be documented once the appropriate clean-up procedure has been implemented. (See Section 9 dealing with spill clean-up.)
Incidents involving genetically modified organisms or quarantine materials resulting in a breach of containment shall be reported to the relevant authorities.
For certain infections, notification of local authorities may be necessary .
Personnel should be encouraged to report all overt exposures or ‘near hits’, so that they may be documented, investigated and, if necessary, procedures changed. This may prevent another or a similar circumstance producing an incident, injury or illness.
NOTE: Appendix B provides an example of an incident/illness reporting form.
An emergency evacuation plan shall be developed in accordance with AS/NZS 2243.1. The plan shall address emergency microbiological issues and shall include minimization of the microbiological risk associated with any emergency evacuation. The plan should take into account the different arrangements for entry and exit associated with the containment level of the facility.
Contingency plans shall be developed for the spillage of microorganisms and breaches of containment caused by the release of microorganisms outside the laboratory through accident, deliberate action, natural disaster, fire, sabotage, theft, or any other event.
A readily accessible first aid kit shall be provided in an unlocked and clearly labelled container. The contents of the kit shall be appropriate to the needs of the laboratory and maintained in a satisfactory condition.
18NOTE: Reference should be made to National, State and Territory legislation for first aid treatment in the workplace.
All work with microorganisms requires the use of standard techniques to minimize risk to people and the environment. Such techniques also maintain the purity of strains of isolates in the laboratory.
Microorganisms vary widely in their ability to infect humans, animals, plants and invertebrates or to spread in the environment. There is obvious, but varied, risk to people from work with microorganisms isolated from or infecting humans. With regard to microorganisms infecting animals, many do not cause human disease, but some zoonotic microorganisms are responsible for serious human infections and can be responsible for serious harm to the economy and the environment.
Some microorganisms that infect arthropods are capable of causing human disease. The arthropods are then said to be vectors of disease, for example, mosquitoes may transmit arboviruses and lice may transmit rickettsiae.
Certain soil microorganisms, while not pathogenic to humans, may cause diseases in plants and be spread to new locations from improper handling or practices. In general, microorganisms from plant and fish diseases rarely infect humans. Certain microorganisms infecting plants or animals are subject to strict quarantine control in Australia and New Zealand, to protect the environment and primary industry.
Certain microorganisms, e.g. Clostridium botidinum, produce small molecules termed toxins that account for their pathogenicity. Some of the microorganisms that produce toxins are listed in Risk Groups 2 and 3. Toxins are also produced by certain plants and animals such as ricin from castor beans and saxitoxin from shell fish. The safety considerations for working with toxins from these sources are not discussed in this Standard.
The basic approach to working with microorganisms is to regard them as potential pathogens and to handle them with standard microbiological techniques which, in the main, protect the environment and the operator and maintain the purity of the strain or isolate.
Microorganisms vary widely in their infectivity. This is partly due to differences in the portal of entry of the organism (e.g. by skin penetration, ingestion, entry via the respiratory tract or entry via the conjunctiva), the physiology of the microorganism, the infectious dose and the ability of the microorganism to overcome intrinsic immune and other defences of the host.
Surveys of the causes of laboratory-acquired infections (see Reference 1.2) have shown that only about 20% of cases followed known accidents with infectious material, the most common being skin penetration accidents, e.g. with a needle and syringe and injury from broken glass. Spillage, mouth pipetting, leakage during centrifugation and bites from infected animals were other causes. Simple precautions can reduce the likelihood of such accidents occurring.
Many of the remaining 80% of infections are believed to be due to inhalation of aerosols that may be produced from common laboratory operations. Such operations include vortexing, sonicating, homogenizing, dropping cultures of high-titre material, blowing out the last few drops in a pipette, removing a needle from a rubber seal, centrifuging, grinding, vigorous shaking or mixing, opening containers of infectious material whose internal pressure may be different from ambient pressure, intranasal inoculation of animals and harvesting of infected tissues from animals and eggs.
19The probability that an aerosol will contain an infectious dose of an organism is broadly related to the concentration (titre) of the organism in the material being handled. The risk is therefore increased when handling bacterial or viral isolates propagated to high titre in culture or in animals, as compared with clinical specimens, food, water and other samples which may contain fewer organisms. Indeed, high titre cultures of some microorganisms (e.g. some arboviruses) may be infectious by the aerosol-respiratory tract route or through broken skin in the laboratory even though in nature they are normally transmitted by insect bite.
Special containment equipment and procedures have been designed to protect laboratory workers from infection with those microorganisms with a ‘track record’ of transmission by the aerosol-respiratory tract route.
Clause 3.2 describes the classification of microorganisms by risk group based, when possible, on past experience with the infectious potential of the microorganism or on microbiologically-informed prudence when a newly-discovered microorganism of uncertain infectious potential has to be handled.
Section 4 provides the principles on which the requirements for containment facilities are based and requirements common to all types of containment laboratories and facilities.
Section 5 sets out the classification of laboratories, physical containment equipment, laboratory design and procedures to be followed when working in laboratories with microorganisms classified at the various risk groups.
Sections 6, 7 and 8 describe the classification of animal, plant and invertebrate facilities, physical containment equipment, facility design and procedures to be followed when working with microorganisms classified at the various risk groups.
Classifications of infectious microorganisms according to degree of risk have been published in the USA, Canada and the UK, together with recommendations for appropriate laboratory facilities for working with them (see References 1.3, 1.4 and 1.5). The World Health Organization (WHO) suggests each country draw up risk groups according to the microorganisms encountered within its boundaries (see Reference 1.6). Unless otherwise stated, references to particular risk groups, e.g. Risk Group 1, refers to human and animal, plant and invertebrate risk groups.
The following classification has been drawn up for microorganisms that are infectious for humans and animals for Australia and New Zealand by modification of the WHO guidelines and is based on the pathogenicity of the agent, the mode of transmission and host range of the agent, the availability of effective preventive measures, and the availability of effective treatment:
The risk grouping of plant infectious microorganisms is primarily concerned with containment of plant pathogens to avoid risk to the environment. Plant pathogens are infectious agents capable of causing disease in plants and include fungi, bacteria, viruses, viroids, rickettsiae, phytoplasmas and nematodes.
Factors considered in relation to the risk from plant infectious microorganisms are—
The following four risk groups are used to manage risks posed by plant infectious microorganisms:
The risks posed by invertebrates are based on the nature of the microorganism that they may be carrying and the nature of the invertebrate itself.
Examples include viruses in mosquitoes, midges and biting flies, Borrelia in soft ticks, trypanosomes in Triatomid bugs, and tospoviruses in thrips.
Factors considered in relation to the risk are—
The following four groups are used to identify the risks posed by infectious microorganisms carried by invertebrates:
Tables 3.1 to 3.8 and 3.9 to 3.11 list examples of microorganisms in Risk Groups 2 to 4 and Plant Risk Groups 2 to 4 respectively. See Clause 3.3.4 in relation to invertebrate risk group examples. The risk group classifications listed in Tables 3.1 to 3.7 are appropriate for small-scale laboratory operations with microorganisms of Risk Groups 2 and 3. Where larger volumes or very high concentrations of the microorganisms are to be handled, the risk of infection or inadvertent release from containment can be higher and additional precautions or an increase in physical containment level may be appropriate. This also applies to situations where the potential to cause environmental and economic damage is a significant concern.
A risk assessment shall be conducted on all microorganisms to determine if the work needs to be conducted with additional precautions or in a higher level of physical containment. The risk assessment should include a review of recent literature to determine if any additional information may warrant changes to the risk grouping or the level of physical containment.
No tables are provided for microorganisms belonging in Risk Group 1, as the number of relevant microorganisms is large.
Tables 3.1 and 3.5 list examples of bacteria of Risk Group 2 and Risk Group 3 respectively and additional information is indicated in footnotes. Currently, no bacteria are classified in Risk Group 4. In addition to the information in the tables, reference should be made to the work practices specified for the relevant level of physical containment.
22Guidelines for Australian Mycobacteriology Laboratories published by the National Tuberculosis Advisory Committee or Guidelines for Tuberculosis Control in New Zealand 2003 published by the Ministry of Health New Zealand, as appropriate, should be consulted for specific requirements for collection, handling and culture of specimens for mycobacteria.
Many parasites are regarded as Risk Group 2, with respect to their infectious stages. Preparations that are known to be free of infectious stages may not require a containment level corresponding to this risk group. Table 3.2 lists examples of Risk Group 2 parasites.
Tables 3.3 and 3.6 list examples of fungi of Risk Group 2 and Risk Group 3 respectively.
Tables 3.4, 3.7 and 3.8 list examples of viruses for Risk Groups 2, 3 and 4 respectively.
The additional containment requirements for poliovirus set out in Appendix C shall be applied.
Tables 3.9, 3.10 and 3.11 list examples of plant pathogens of Plant Risk Groups 2, 3 and 4 respectively.
Invertebrates are able to act as vectors for human, animal and plant disease. Pathogens that use invertebrates as vectors are not listed in separate tables. Instead, examples of pathogens of Risk Groups 2, 3 and 4 that are vectored by invertebrates are included in Tables 3.1, 3.2, 3.4.3.5 and 3.7 to 3.11.
Such specimens would normally be regarded as Risk Group 2 and shall be handled in Physical Containment Level 2 facilities unless a higher risk group is indicated by the clinical notes. This applies in all microbiology and other pathology laboratories, e.g. for haematology and biochemistry. However, if a microorganism of a higher risk group is isolated from a specimen, the isolate and all samples from that source shall be handled according to the corresponding risk group, and at the appropriate physical containment level.
All clinical and diagnostic specimens shall be treated with care as they may contain multiple types of infectious microorganisms. Examples of precautions that should be adopted are provided in the Department of Health and Ageing publication. Infection control guidelines for the prevention of transmission of infectious diseases in the health care setting and NOHSC: 2010.
Cultures and materials containing microorganisms are regularly transferred within and between institutions and PC levels. Laboratory-acquired infections have occurred because of cross-contamination and ineffective attenuation or inactivation.
It is strongly recommended that prior to despatch and upon receipt of ‘pure’ cultures, tests are carried out to ensure purity.
If infectious microorganisms are attenuated or inactivated prior to removal to a lower PC level or prior to transfer between institutions, the attenuation or inactivation processes shall
23be verified. The identity and purity of cultures shall be confirmed before they are transferred to lower containment levels or between institutions.
Routine quality control testing of registered live vaccine strains should be carried out in a BSC.
Work with cells has the potential to be hazardous to laboratory workers and the environment, depending on the source of the cells and the likelihood that they contain infectious microorganisms. In some instances, PCI teaching labs can be adequate if good microbiological practices are followed, e.g. work with standard human cell lines. However, the preparation of primary cells from human organs or tissues shall be conducted in PC2 containment. The manipulation of these cell lines should be done in Class II BSCs. Some cell lines contain Mycoplasma and although they can be ‘cleaned up’, can become reinfected and again pose a hazard to the laboratory worker. Cell lines from an animal source can also contain microorganisms that are capable of causing disease in humans and animals.
Plant cells and tissue cultures can contain plant infectious microorganisms that have the potential to spread in the environment if inadvertently released and cause economic and environmental damage.
A documented risk assessment shall be carried out to determine what level of containment is required for the cells proposed for use.
All cells shall be decontaminated before disposal.
Prions are resistant to most traditional methods of inactivation used for other microorganisms such as formaldehyde, ultraviolet light, ethylene oxide, ionizing radiation and moist heat at 121°C. Because of the difficulties in inactivating the infectivity, these agents pose particular laboratory problems. However, they are not easily spread from host to host and the usual mechanism of spread appears to be by the ingestion or grafting of infectious material. When working with infectious or potentially infectious prions, a laminar flow cytotoxic drug safety cabinet shall be used. Table 3.4 lists examples of prions of Risk Group 2. See also Clauses 10.8 and 12.2.1. Table F2 provides guidance on disinfection of equipment or surfaces contaminated with prions.
24| Organism |
|---|
| Abiotrophia spp. |
| Acidovorax spp. |
| Acinetobacter spp. |
| Actinobacillus spp. |
| Actinomyces spp. |
| Aeromonas hydrophila |
| Afipia spp. |
| Arcanobacterium haemolyticum |
| Bacillus cereus |
| Bartonella henselae, B. quintana, B. vinsonii, B. elizabethiae, B. weisii |
| Bordetella pertussis |
| Borrelia (mammalian) spp. |
| Brucella ovis |
| Brucella spp. serology only |
| Burkholderia spp. (except B. mallei), Burkholderia pseudomalleib,f |
| Campylobacter coli, C. fetus, C. jejuni |
| Capnocytophaga canimorsus |
| Chlamydia spp. (except C. psittaci) |
| Clostridium spp. |
| Corynebacterium diphtheriae, C. renale, C. pseudotuberculosis |
| Coxiella burnetii serology, other tests on samples |
| Dermatophilus congolensis |
| Edwardsiella tarda |
| Eikenella corrodens |
| Enterococcus spp. (Vancomycin-resistant strains) |
| Erysipelothrix rhusiopathiae |
| Pathogenic Escherichia coli (except genetically rippled strainsc) |
| Verocytotoxin-producing Escherichia coli (VTEC)b |
| Fusobacterium spp. |
| Gardnerella vaginalis |
| Gordona spp. |
| Haemophilus influenzae, H. ducreyi |
| Helicobacter pylori |
| Kingella kingae |
| Klebsiella spp. |
| Legionella spp. |
| Leptospira interrogans (all serovars)d |
| Listeria spp., Listeria monocytogenese |
| Moraxella spp. |
| Mycobacterium spp. other than M. tuberculosis complexf |
| Mycobacterium tuberculosis complex (except multi-drug resistant strainsf, g, h) |
| Mycoplasma pneumoniaef |
| Neisseria gonorrhoeae, Unspeciated Neisseriab, f, N.meningitidisb, f |
| Nocardia spp. |
| Oligella spp. |
| Pasteurella spp. |
| Pseudomonas spp. |
| Rhodococcus equi |
| Salmonella serovars |
| Salmonella Paratyphi A and Bb |
| Salmonella Typhib, e |
| Serratia spp. |
| Shigella spp.b |
| Sphaerophorus necrophorus |
| Staphylococcus aureus |
| Stenotrophomonas maltophilia |
| Streptobacillus moniliformis |
| Streptococcus pyogenes, S. pneumoniae |
| Treponema pallidum |
| Ureaplasma ureolyticum |
| Vibrio cholerae, V. parahaemolyticus, V. vulnificus |
| Yersinia spp. (except Y. pestis) |
| a This list is not exhaustive. Some species of some genera may be classified as Risk Group 1 subject to a risk assessment and check of current literature. b Low infectious dose, high pathogenicity, common source of laboratory-acquired infections. c For genetically crippled strains, refer to the gene technology regulations. d Can penetrate intact skin. e May be dangerous for pregnant women. f High risk of aerosol spread. g Vaccination, see Clause 2.6.4. h Less than 5000 cultures per year. See references in Clause 3.3.2.1. |
| Organism |
|---|
| Ancylostoma duodenale |
| Ascaris lurmbricoides |
| Babesia divergens |
| Babesia microti |
| Brugia spp. |
| Clonorchis sinensis |
| Cryptosporidium spp. |
| Echinococcus spp. |
| Entamoeba histolytica |
| Giardia duodenalis (also known as Giardia lamblia and Giardia intestinalis) |
| Hymenolepis diminuta |
| Hymenolepis nana |
| Leishmania (mammalian) spp. |
| Loa loa |
| Naegleria fowleri |
| Necator americanus |
| Opisthorchis spp. |
| Plasmodium (human and simian) |
| Strongyloides stercoralisb |
| Taenia saginata |
| Taenia soliumc |
| Toxocara canis |
| Toxoplasma gondiid |
| Trichinella spiralis |
| Trypanosoma brucei subspp. |
| Trypanosoma cruzi |
| Wuchereria bancrofti |
| a This list is not exhaustive. b Filariform larvae may cross intact skin. c Accidental ingestion of eggs may lead to cysticercosis d May be teratogenic |
| Organism |
|---|
| Aspergillus fumigatus and A. flavus |
| Candida albicans |
| Cladophialaphora spp. |
| Cryptococcus gattii |
| Cryptococcus neoformans |
| Epidermophyton floccosam |
| Microsporum spp. |
| Scedosporium spp. |
| Sporolhrix schenckii |
| Trichophyton spp. |
| a This list is not exhaustive. |
| Virus or prion |
|---|
Adenoviridae
|
Arenaviridae
|
Caliciviridae
|
Coronaviridae
|
Flaviviridae
|
Hepadnaviridae
|
Herpesviridae
|
Orthomyxoviridae
|
Paramyxoviridae
|
Parvoviridae
|
Picornaviridae
|
Poxviridae
|
Prions
|
Reoviridae
|
Retroviridae (serology, other tests on samples)
|
Togaviridae
|
Unclassified
|
| a This list is not exhaustive. b While these agents are exotic to Australia, the AQIS permit determines the level of containment required. c Vaccination available, see Clause 2.6.4. d May be teratogenic. e See also Tables 3.7. f May be dangerous for pregnant women. NOTE: Hepatitis G and hepatitis TT have been excluded from this Table as there is insufficient evidence that these agents are associated with disease. |
| Organism |
|---|
| Bacillus anthracis |
| Bartonella bacilliformis |
| Burkholderia mallei |
| Brucella spp. (except serology (see Table 3.1) and B. ovis) |
| Chlamydia psittaci |
| Coxiella burnetii (cultures, animal work and concentrates)b,c |
| Francisella tularensis (type A) |
| Mycobacterium tuberculosis complexc,d,e |
| Rickettsia spp. |
| Yersinia pestis |
| a This list is not exhaustive. b May be dangerous for pregnant women. c Vaccination, see Clause 2.6.4. d Respiratory protection should be considered. e Greater than 5000 cultures per year, susceptibility testing, known multi-drug resistant strains. See references in Clause 3.3.2.1. |
| Organism |
|---|
| Blastomyces dermatitidis |
| Coccidioides immitisb |
| Coccidioides posadasii |
| Histoplasma spp. |
| Paracoccidioides brasiliensis |
| Penicillium marneffei |
| a This list is not exhaustive. b May be dangerous for pregnant women. NOTE: The mycelial forms of these fungi produce highly infectious conidia. The use of plate cultures should be avoided. |
| Virus |
|---|
Arenaviridae
|
Bunyaviridae
|
Coronaviridae
|
Flaviviridae
|
Orthomyxoviridae
|
Paramyxoviridae
|
Retroviridae (from cultures and concentrates)
|
Rhabdoviridae
|
Togaviridae
|
| a This list is not exhaustive. b Animal inoculations to be performed under PC4 containment. c While these agents are exotic in Australia, the AQIS permit determines the level of containment required. d Vaccination available, see Clause 2.6.4. |
| Virus |
|---|
Arenaviridae
|
Bunyaviridae
|
Filoviridae
|
Flaviviridae
|
Herpesviridae
|
Paramyxoviridae
|
| a This list is not exhaustive. b Although only a few cases of infection with Hendra have occurred, the death rate has been high. It is considered appropriate to include this virus in Risk Group 4 from the limited information available. |
| Organism |
|---|
| Grapevine fan leaf nepovirus |
| Asparagus stem blight (Phomopsis asparagi) |
| Tomato yellow leaf curl virus |
| Citrus tristeza virus |
| Onion smut (Urocystis cepulae) |
| Lettuce leaf blight (Pythium tracheiphilum) |
| a This list is not exhaustive. |
| Organism |
|---|
| Citrus canker (Xanthomonas axonopodis) |
| Fire blight (Erwinia amylovora) |
| Plum pox potyvirus |
| Potato cyst nematode (Globodera pallida) |
| Pierce’s disease (Xylella fastidiosa) |
| Chestnut blight (Cryphonectria parasitica) |
| Pine pitch canker (Fusarium circinatum) |
| a This list is not exhaustive. |
| Organism |
|---|
| Guava rust (Puccinia psidii) |
| Karnal bunt (Tilletia indica) |
| Sudden oak death (Phytophthora ramorum) |
| Potato leaf blight (Phytophthora infestans exotic strains) |
| Grapevine rust (Phakopsora euvitis) |
| a This list is not exhaustive. |
Containment of microorganisms involves a combination of buildings, engineering function, equipment, and worker practices to handle microorganisms safely. Physical containment is the term used to describe procedures and structures designed to reduce or prevent the release of viable organisms into the outside environment. Four PC levels, PC1 to PC4. are assigned for work with microorganisms.
Viable microorganisms and animals, plants or invertebrates inoculated with microorganisms from defined risk groups shall be used, stored or housed in corresponding or higher level containment facilities.
The three general descriptors by which microbiological containment is achieved are known as primary, secondary and tertiary containment measures. Optimal microbiological containment is provided by the ‘box-within-a-box’ principle (see Figure 1), where the highest hazards are enclosed by multiple containment measures.
Primary containment measures are the constraints immediately surrounding the source of infectious material, such as a BSC, a ventilated animal enclosure, a sealed animal room with appropriate air pressure controls, or the leakproof container forming the inner receptacle of an approved I ATA infectious materials transport container. Invariably, there is a primary barrier or other containment measure restricting the passage of infectious microorganisms.
Secondary containment measures include the design of a laboratory or device that encloses the primary containment. Facility design and engineering operations providing laboratories with air pressure control and directional air flow (supplemented by HEPA filtration of exhaust air) are examples of secondary containment measures. Another example is the secondary receptacle of an approved I ATA transport container. In the laboratory or animal room, secondary physical containment measures are invariably supplemented by defined work practices, including PPE.
Tertiary containment measures provide protection of the wider environment by supporting the secondary containment, e.g. using the outer packaging of an approved IATA transport container, an isolated building complex, control of people movements, and provision of support services such as decontamination and laundering of clothing and disposal of infectious wastes.
35The hazard and risk posed by different microorganisms varies greatly and this is reflected by the organization of microorganisms into the risk groups described in Section 3. The physical containment level used when working with microorganisms shall be at least the appropriate level for the risk group of the microorganism, i.e. Physical Containment Level 1 for Risk Group 1, Physical Containment Level 2 for Risk Group 2. Unless otherwise stated, Risk Group 1 to Risk Group 4 mean human and animal, plant and invertebrate Risk Group 1 to Risk Group 4 and PC 1 to PC4 mean laboratory, animal, plant and invertebrate PC 1 to PC4.
All work done in a laboratory or facility of a specific level shall follow procedures prescribed for that level of physical containment.
Section 5 details the appropriate requirements and recommendations for four physical containment levels of laboratories corresponding to Risk Groups 1 to 4 defined in Clause 3.2.2 and include the laboratory structural requirements and facilities, PPE, safety equipment, practices, techniques and health monitoring procedures. The four classifications of laboratories are defined by the physical containment prefix ‘PC’.
Sections 6, 7 and 8 contain the corresponding requirements for animal, plant and invertebrate facilities. The corresponding classifications for animal, plant and invertebrate facilities have ‘Animal’, ‘Plant’ or ‘Invertebrate’ preceding ‘PC’, as appropriate.
A PC1 laboratory or facility is suitable for work with microorganisms where the hazard levels are low, and where laboratory or facility personnel can be adequately protected by standard laboratory practice. This level of laboratory or facility with its practices and equipment is usually suitable for student and undergraduate teaching laboratories. The organisms used should generally be classified as Risk Group 1. Specimens that have been inactivated or fixed may be handled in PC 1 facilities.
This level of laboratory or facility with its practices and equipment is applicable to research, diagnostic and other premises where work is carried out with microorganisms or material likely to contain microorganisms that are classified as Risk Group 2 microorganisms. If working with specimens containing microorganisms transmissible by the respiratory route or if the work produces a significant risk to humans or the environment from the production of infectious aerosols, a biological safety cabinet shall be used.
This level of laboratory or facility with its practices and equipment is applicable to research, diagnostic and other premises where work is carried out with microorganisms or material likely to contain microorganisms that are classified as Risk Group 3 microorganisms.
A PC3 laboratory or facility provides additional building features and services to minimize the risk of infection to individuals, the community and the environment.
This level of laboratory or facility with its practices and equipment is applicable to work with microorganisms classified as Risk Group 4 microorganisms.
36A PC4 laboratory or facility is situated in a building separate from other laboratories or facilities or constructed as an isolated area within a building. The facility is maintained under negative pressure and includes secondary barriers such as sealable openings, airlocks or liquid disinfection barriers, a clothing-change and shower room contiguous to the laboratory or facility ventilation system, and exhaust air and liquid waste decontamination systems to prevent the escape of microorganisms to the environment.
A PC4 laboratory or facility may be of two types; one where work is conducted in a Class III biological safety cabinet exhausting outside the facility or one where the work is conducted without being isolated in such a manner and staff wear fully encapsulated positive pressure suits.
The design of the facility shall take into account the potential impact of severe environ mental and climatic events (such as seismic events, flooding, snow, wind storms, fire, cyclones and hail storms) that are likely to occur in the area in which it is located so that the risk of damage to the containment barrier is minimized.
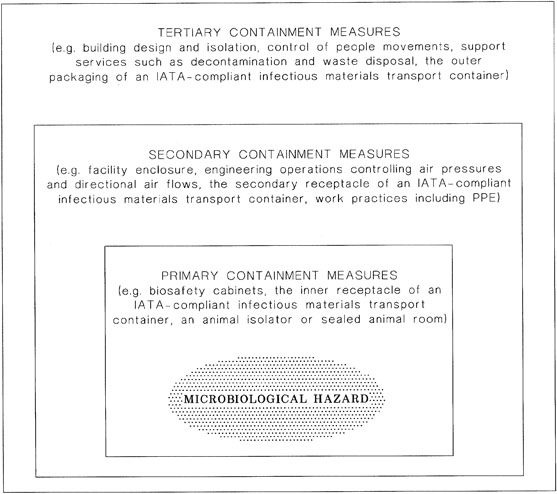
FIGURE 1 RELATIONSHIP BETWEEN CONTAINMENT MEASURES
37The physical containment level used when working with microorganisms in laboratories shall be at least the appropriate level for the risk group of the microorganism, as set out in Section 4. Requirements for laboratory PC1, PC2, PC3 and PC4 containment facilities are set out in Clauses 5.2, 5.3, 5.4 and 5.5 respectively.
All work done in a laboratory of a specific level shall follow procedures prescribed for that level of physical containment.
A Laboratory PC1 facility, in which laboratory personnel can be adequately protected by standard laboratory practice and no containment equipment is required, is suitable for work with microorganisms in Risk Group 1. Specimens that have been inactivated or fixed may be handled in a PC1 laboratory.
This level of facility with its practices and equipment is appropriate for student and undergraduate teaching laboratories. Work may be carried out on the open bench.
A sign complying with Appendix D showing the level of containment, together with hazard symbols as appropriate and any access restrictions should be prominently displayed at the entrance.
Laboratory facilities shall be constructed in accordance with AS/NZS 2982.1 and the following requirements to facilitate microbiological safety and reduce the likelihood of microorganisms escaping from containment:
Laboratory personnel shall observe the work practices in AS/NZS 2243.1 as well as the following (see also Clause 2.1.6):
NOTE: This includes offices within the containment facility boundary.
Protective clothing to afford protection to the front part of the body shall be worn within the laboratory.
NOTE: A rear-fastening gown is preferable.
NOTE: Disposable loops may be used as an alternative.
NOTES:
A Laboratory PC2 facility is suitable for work with microorganisms in Risk Group 2 and incorporates all the requirements of a Laboratory PC 1 facility with additional requirements relating to conditions of access, safety equipment and staff training requirements. With good microbiological techniques, work with these microorganisms may be carried out on the open bench. If working with specimens containing microorganisms transmissible by the respiratory route or if the work produces a significant risk from the production of infectious aerosols, a biological safety cabinet shall be used.
Institutions with large areas such as entire floors, multiple floors or multiple buildings designated as PC2 areas shall ensure that safety is maintained for both laboratory workers and non-laboratory persons. There is a need to restrict access to the PC2 areas, including via lifts and stairs, preventing the use of laboratories as thoroughfares and ensuring that eating and drinking is prohibited in the entire PC2 area.
Where lifts operate through multiple PC2 levels, the lift itself shall not be classified as PC2 as it may be used by non-laboratory persons. The lift shaft and lift motor areas are required to be accessed by lift technicians and cannot be readily decontaminated. Where it is required to use the lift to transport infectious materials from one floor to another, e.g. to a pressure steam sterilizer on another floor, the infectious materials shall be either double-bagged, the outer bag of which should be sealed during transport, or placed in a secondary sealable, unbreakable container for the transport.
Potentially contaminated laboratory gowns and gloves shall not be worn in lifts.
Inward air flow to the PC2 areas from lifts and lift shafts shall be maintained. This requires attention to cater for the pressure fluctuations and air movements caused by movement of the lift car in the shaft (‘piston effect’).
Where lift equipment is accessed via PC2 areas, care shall be taken to ensure that lift maintenance personnel are able to perform their work without compromising safety or containment.
Stairs that connect only to PC2 areas may be classified as PC2 spaces provided that they satisfy the PC2 construction requirements. Users shall be made aware that wearing potentially contaminated laboratory gowns and gloves in stairs can be a source of cross-contamination. Infectious materials shall be double-bagged, the outer bag of which should be sealed during transport, or placed in a secondary sealable, unbreakable container for transport.
Where large areas are classified as PC2, including associated write-up areas, the prohibition of eating and drinking applies in these adjacent areas. See also Clause 5.3.3(i).
40In addition to the construction requirements specified for PC1 laboratories in Clause 5.2.2, the following shall apply:
NOTES:
NOTE: Structural joints should be minimized in containment laboratories.
NOTE: Where large spaces, such as ceiling voids, are used as part of return or exhaust air paths, care should be taken by personnel accessing such spaces due to the potential for long term build-up of laboratory dust.
NOTE: The pressure steam sterilizer should be as close to the laboratory as possible.
NOTE: Gowns should be hung or stored individually to prevent cross-contamination or contamination of the inside of gowns.
NOTE: Implementation of the second option will generally preclude the use of tiled ceilings for such laboratories.
41NOTE: Where freezers or refrigerators are used by multiple personnel, it is recommended that the names and telephone numbers of the users are displayed on the front of the unit.
An inward flow of air shall be maintained by forced extraction of laboratory air to minimize the spread of aerosols in the event of an inadvertent spill. Recirculation is permitted but not into areas outside the PC2 facility.
Recirculated air shall be filtered to remove airborne particulates. Where long term build-up of particulate material can be hazardous to personnel, filtration should occur before air leaves the laboratory, i.e. at or below ceiling level.
Ventilation system components such as filters and filter plenums can accumulate particulates. Any special precautions that are required for maintenance personnel should be noted at points of access to this equipment.
Ventilation air shall not be directed towards doors or located in positions that can disturb air flow at BSCs.
NOTE: A risk assessment should be conducted to determine the duration of operating hours of the ventilation system based on the active work hours and the ongoing use of equipment such as incubators, water baths and warm rooms.
A Class I or II biological safety cabinet (see Clause 10.7) shall be provided if work with microorganisms transmissible by the respiratory route or work producing a significant risk from aerosol production is anticipated.
Installation and use, including the decontamination of the biological safety cabinet, shall be performed in accordance with the requirements of AS/NZS 2647.
A laminar flow cytotoxic drug safety cabinet (see also Clause 10.8) shall be provided if work involving prions is intended.
Installation and use, including the decontamination of the safety cabinet shall be performed in accordance with the requirements of AS 2639.
When infectious materials are used, a centrifuge fitted with either sealed rotors or sealed buckets shall be used. (See also Clause 10.3.)
In addition to the work practices described in Clause 5.2.3 for PC1 laboratories, the following work practices shall be observed:
NOTES:
NOTE: Large items of equipment can interfere with the airflow pattern in a Class II BSC and correct operation of the cabinet should be validated with the equipment in situ. See Clause 10.3.1.
NOTE: This has been a common cause of laboratory acquired infections.
NOTE: Thermal decontamination of pipettes that are not fully immersed in a liquid, i.e. are empty, can only be achieved in a pre-vacuum steam sterilizer.
43NOTES:
NOTE: Appendix F provides information on disinfectants.
A Laboratory PC3 facility is suitable for work with infectious microorganisms in Risk Group 3 and incorporates all equipment and practices for Physical Containment Levels 1 (Clause 5.2) and 2 (Clause 5.3); however, additional conditions of access, safety equipment and staff training apply.
NOTE: The design of a PC3 facility is complex and those planning its construction should seek specialized advice. See also Appendix G for examples of recommended layouts for PC3 facilities showing the design principles involved and Appendix H for airtightness considerations.
In addition to construction requirements described for PC 1 and PC2 in Clauses 5.2.2 and 5.3.3, the following shall apply:
NOTES:
NOTE: Examples of suitable mechanisms include entry and egress ‘traffic light’ alarm systems, door interlock control systems or viewing panels if suitable.
NOTE: Examples of suitable arrangements are the viewing panels in doors required in Item (e) if they allow adequate viewing of laboratory occupants, viewing panels in walls or electronic visual monitoring facilities (e.g. viewing cameras or closed circuit television).
NOTES:
NOTE: The design of the facility should avoid inaccessible spaces. See Appendix H for recommendations on design for airtightness and periodical retesting.
NOTE: See Appendix H.
Some institutions have PC3 facilities consisting of several laboratories within the negative pressure area.
Additional safety structures that may be incorporated into the barrier wall include a dunk tank for removal of containers and materials that can withstand immersion in liquid disinfectant, a decontamination chamber for gaseous decontamination or introduction of large items of equipment and a pass-through box for entry of materials into the facility.
A ventilation system that establishes a negative pressure in the laboratory shall be provided so that there is a directional airflow into the working area. Where laboratories have supply air systems, the supply air and exhaust air systems shall be interlocked, to ensure inward airflow at all times. The proper directional airflow into the laboratory shall be verified by airflow tests. The laboratory (including the airlock) shall be structurally designed to take account of the operation under negative pressures.
Failure of a single component, such as an exhaust fan or a supply fan, can result in extremely high positive or negative pressures in the laboratory. Alarms and failure mode operations of ventilation systems shall address this risk to ensure that interlocks operate rapidly to stop systems. The laboratory shall be constructed to withstand, without cracking or deterioration, the maximum positive and negative pressures that can be generated until failure mode safeguards operate. Automatic and manual failure mode sequences shall be independent of any automated control system that may, itself, be the primary cause of a failure situation.
All air that leaves the laboratory shall be exhausted in accordance with the requirements of this Clause.
Air may be recirculated within each laboratory. If air is recirculated, this shall be achieved utilizing internally-mounted airconditioning equipment such as fan coil units and split system airconditioning units. Any internally-mounted equipment shall be provided with removable panels as required to ensure the complete penetration of gas or vapour during room decontamination.
NOTES:
Ventilation equipment and outlets shall be located to minimize the disturbance to the open faces of Class I and Class II biological safety cabinets.
The laboratory ventilation shall incorporate the following features:
NOTE: Additional precautions should be considered when one or more surfaces are external and exposed to fluctuations of pressure due to wind effect. Suitable precautions include the use of an interstitial space that can be maintained at the zero reference pressure and the use of solid walls such as concrete with minimal joints, which are unlikely to be perforated or leak.
NOTE: A variable speed drive on the exhaust fan is preferred to facilitate room pressure control adjustments.
NOTE: The prefilter may be installed in the exhaust HEPA filter housing or in the laboratory. Installation within the laboratory can facilitate access and changing.
The emergency stop button shall operate independently of the main ventilation control and main laboratory pressure control system such that emergency isolation of the ventilation can be implemented in event of central control system malfunction.
NOTES:
Access to voids surrounding the immediate perimeter of the laboratory and to the ventilation equipment that serves the laboratory shall be restricted to authorized persons. Items of equipment, ducts and access panels to contained sections of the ventilation system shall be marked with biohazard labels to minimize the risk of accidental exposure to air or to contaminated surfaces. The installation of services shall ensure proper access to equipment such as HEPA filters for maintenance and testing personnel and their equipment.
In addition to equipment specified for PC2 (Clause 5.3.5), the following shall be provided:
NOTE: The provision of an uninterruptible power supply should be considered for BSCs.
In addition to work practices specified for PC1 (Clause 5.2.3) and PC2 (Clause 5.3.6), the following practices shall be observed:
NOTE: In most circumstances, the appropriate removal procedure is removing the gloves then decontaminating the hands followed by removal of eye protection, gown and respiratory protection, taking care not to touch potentially contaminated parts of PPE when doing so, then decontaminating hands again.
NOTE: Suitable measures include the use of dedicated facility footwear, the use of overshoes or a combination of these measures.
See Clause 2.6.
A Laboratory PC4 facility is suitable for work with infectious microorganisms in Risk Group 4 and incorporates all equipment and practices for PC1, PC2 and PC3 (Clauses 5.2, 5.3 and 5.4); however, additional requirements on conditions of access and egress, safety equipment and staff training apply .
A PC4 laboratory may be of two types; a laboratory where work is conducted in a Class III biological safety cabinet exhausting outside the laboratory or one where the work is conducted without being isolated in such a manner and staff wear fully encapsulated positive pressure suits.
NOTE: The design of a PC4 facility is complex and those planning its construction should seek specialized advice. See also Appendix G for examples of recommended layouts for PC4 facilities showing the design principles involved and Appendix H for airtightness considerations.
In addition to the design features and facilities specified for PC1, PC2 and PC3, the following facilities shall be provided:
49NOTE: Recommendations on acceptable room airtightness are given in Appendix H.
NOTE: A security card access procedure, with additional numerical pad or biometric access control, is preferred as a means of entry.
The outer shower door shall form the laboratory containment boundary for decontamination purposes.
The four doors of each entry/exit path shall raise an alarm if left open.
An entry and egress ‘traffic light’ alarm system or door interlock control system shall be provided to prevent the simultaneous opening of the doors on each side of the shower.
NOTES:
For certain requirements, a specially designed suit area may be provided within the facility. Personnel who enter this area shall wear a one-piece positive pressure suit that is ventilated by a life support system. If provided, positive pressure suit areas shall comply with the following additional requirements:
The facility ventilation shall comply with the following:
The supply air HEPA filter shall prevent the outflow of contaminated air if air pressures become unbalanced within the facility.
In addition to the requirements in Clause 5.5.3.1, positive pressure suit areas shall comply with the following:
NOTE: A 25 Pa differential is recommended.
For work with agents of Risk Group 4, one of the following shall be provided:
In addition to the work practices specified for Physical Containment Levels 1 (Clause 5.2.3), 2 (Clause 5.3.6) and 3 (Clause 5.4.7), the following practices shall be observed:
NOTE: Containers may be opened in non-PC4 laboratories only if the biological material has been rendered non-infectious or non-toxic, and the space in the primary and secondary containers has been decontaminated.
A primary container holding viable or intact biological materials shall be opened only in a Class III BSC in another PC4 laboratory.
NOTE: Containers may be opened in non-PC4 laboratories only if the biological material has been rendered non-infectious or non-toxic, and the space in the primary and secondary containers has been decontaminated.
NOTE: The presence of a co-worker either inside the laboratory or observing the work from outside the laboratory should be considered.
In addition, the following work practices apply for positive pressure suit areas:
See Clause 2.6.
54This Section sets out requirements to ensure that animals that are infected with or that may contain infectious microorganisms are contained in facilities that will prevent the escape of the animals and the microorganisms. The general principles of animal containment can also be applied to animals that do not contain any infectious microorganisms, such as specific pathogen free (SPF) animals and genetically modified or transgenic animals. This Section is not intended to be used as a substitute for other regulations or guidelines that apply to these animals, such as those issued by AQIS, OGTR or equivalent New Zealand regulatory agencies.
In Australia, animals exposed to exotic microorganisms shall be housed in containment facilities that meet the requirements of AQIS. Animals exposed to genetically modified microorganisms shall be housed in accordance with OGTR requirements. Disposal of such animals shall be in accordance with the relevant regulations or guidelines.
In New Zealand, animals exposed to exotic microorganisms and animals exposed to genetically modified microorganisms shall be housed in facilities approved by MAF. Disposal of such animals shall be in accordance with the relevant regulations or guidelines.
Facilities and arrangements for animal husbandry and management shall be consistent with good animal welfare practices and in accordance with either the Australian code of practice for the care and use of animals for scientific purposes or the New Zealand Animal Welfare Act 1999, as appropriate.
NOTE: The Guidelines to promote the wellbeing of animals used for scientific purposes: The assessment and alleviation of pain and distress in research animals should also be consulted.
Animals can be held in a variety of containment facilities that are designed to ensure that the animals, and the microorganisms that may be being used in conjunction with the animals, do not escape from containment. Animals under experiment may be either small laboratory animals (e.g. mice or rabbits) or large domestic animals (e.g. pigs, sheep or cattle). The requirements for housing and maintenance of the animals may differ in scale as a result but the overall principles that apply are the same. While some Animal PC 1 facilities confine larger animals in a fenced enclosure, other Animal PC 1 facilities are designed to contain smaller animals such as rodents.
Facilities may be designed and constructed in the same way as a laboratory and may be integral to, and inseparable from, the laboratory itself. At lower containment levels (Animal PC 1 and Animal PC2), there may be little difference between the design and construction of animal and laboratory containment facilities.
Where smaller animals may be infected with, or exposed to, Risk Group 3 or Risk Group 4 microorganisms, it is preferable that they are kept in some form of primary containment device, such as ventilated cages fitted with exhaust HEPA filters. The use of primary containment devices should be considered at all levels of animal facilities to prevent cross-contamination and to prevent exposure of personnel to allergens and microorganisms.
In cases where it is not possible to keep animals in primary containment devices (e.g. for cattle and sheep), or the animal cages or enclosures do not prevent the spread of aerosols, the room itself will form the primary containment. In this situation, in addition to the room exhaust HEPA filters, other measures such as additional construction requirements, specialised PPE, work practices and training may be required to ensure the protection of
55both human health and the environment. Measures for consideration include the use of dedicated PPE that remains in the facility and showering of personnel before leaving the facility.
Waste shall be segregated, decontaminated where necessary and disposed of according to applicable regulations. (See also Section 12.) Animal containment facilities should have access to decontamination facilities within their own areas. Waste from low level (PC1 and PC2) animal containment facilities can be decontaminated outside the facility. However, waste shall be contained to prevent dissemination of any infectious microorganisms. Waste from higher level animal containment facilities shall either be pressure steam sterilized in the facility or decontaminated in a closed system to ensure that all infectious microorganisms are destroyed.
As a general principle, the biological and physical containment recommended for working with infectious agents in vivo and in vitro are comparable. Infected animals should only be handled by trained staff using procedures designed to protect staff and the environment from exposure to the microorganisms. When housing animals in which microorganisms are to be used, the physical containment levels for work with microorganisms shall follow the containment levels appropriate for the microorganism. Requirements for Animal PC1, Animal PC2, Animal PC3 and Animal PC4 facilities are set out in Clauses 6.4, 6.5, 6.6 and 6.7.
Prior to designing facilities, separate areas should be considered for different activities, for example for animal housing, experiments, post-mortem examinations, disposal of wastes and associated maintenance.
Infected, non-infected and quarantined animals should be separately housed, and precautions taken to prevent cross-infection. Even animals that have not been deliberately infected may harbour organisms that are dangerous to humans.
Training staff in animal handling is the best method of preventing injury, both to staff members and to animals (see the Australian code of practice for the care and use of animals for scientific purposes or the New Zealand Animal Welfare Act 1999, as appropriate).
Exposure to animals or animal products (scurf, dander, hair or urine components) can cause allergies and asthma. About 33% of animal handlers have allergic symptoms (e.g. rhinitis) and approximately 10% have animal-induced asthma. Inhalation is one of the most common ways for allergens to enter the body. Some workers develop allergic symptoms fairly quickly, while others can take longer to become sensitized (usually within three years). (See Reference 1.8.) To reduce the incidence of these conditions, adequate ventilation, including an increased number of air changes per hour, should be ensured and local exhaust systems provided where necessary (see Clause 6.3.3). In addition, animal handlers, technical and scientific staff should take appropriate precautions to prevent the development of allergies.
It is recommended that respiratory protection is worn to prevent the development of laboratory animal allergies. Usually P2 particulate respirators are adequate but fit testing of the respirator is important to ensure that it is appropriate for the individual and advice from an occupational hygienist or similar should be sought.
Any unusual personal reaction or allergy to animals or animal products should be reported so that appropriate action can be taken.
56Animal containment facilities require relatively high rates of fresh air ventilation to control odours and contaminants such as animal detritus and ammonia (from waste products). The fresh air ventilation rate shall be sufficient to keep odour and contaminant levels below acceptable threshold limits for the long-term exposure of personnel.
NOTES:
| Animal housing | Fresh airflow (air changes per hour) |
|---|---|
| Open cages, i.e. the room forms the primary containment barrier | 15 |
| Isolators fitted with HEPA exhaust filtration and activated carbon or equivalent odour controlling mechanisms | 12 |
| Isolators fitted with HEPA exhaust filtration and exhaust air is completely removed from the occupied space by capture hood or direct-ducting | 8 |
Infectious bedding, cage wastes and cages from small animals shall be decontaminated prior to disposal or reuse as described in Section 12. Infected carcasses shall be decontaminated prior to disposal. This may be achieved by methods such as alkali digestion, autoclaving, incineration or rendering. All instruments and containers that have been used in procedures with infectious microorganisms should be decontaminated before cleaning. Any special precautions that are needed, such as decay of radioisotopes, should be taken.
NOTE: Decontamination requirements apply for Animal PC2 and higher facilities. See Clauses 6.5 to 6.7.
Where it is necessary to transport animals or animal tissues from the containment facility, the appropriate precautions shall be determined. Tissues fixed to inactivate infectious materials may be removed from the facility. Live animals and animal tissues shall not be moved to a facility of a lower level of containment, e.g. from PC3 to PC2. (See Clause 3.5.)
Post-mortem examinations of animals that are infected or suspected to be infected with pathogenic microorganisms shall be carried out under physical containment conditions equivalent to the risk group of the microorganism present or suspected to be present.
During post-mortems, appropriate PPE such as gloves, aprons and eye protection should be worn. Where there is a risk of infection by the respiratory route, respiratory protection shall be used.
57An Animal PC 1 facility is suitable for work with microorganisms in Risk Group 1 and uninfected animals. Microbiological containment is generally addressed by good work practices.
A sign complying with Appendix D showing the level of containment, together with hazard symbols as appropriate and any access restrictions should be prominently displayed at the entrance.
Animal PC1 facilities shall comply with the following:
NOTES:
NOTE: This option is only for Animal PC1 and Animal PC2 facilities, provided the room is not the primary containment measure.
The work practices for Animal PC 1 facilities shall be as follows:
An Animal PC2 facility is suitable for work with infectious microorganisms in Risk Group 2 and incorporates all the requirements of an Animal PC1 facility with additional requirements of construction, access, safety equipment and staff training.
In addition to the construction requirements specified for Animal PC1 facilities in Clause 6.4.2, the following shall apply:
An inward flow of air shall be maintained by forced extraction of air to minimize the spread of aerosols in the event of an inadvertent spill. Air shall not be recirculated unless animals are kept in primary containment devices that are separately exhausted. If air is recirculated, it shall not be supplied to areas outside the Animal PC2 facility.
Ventilation air shall not be directed towards doors or located in positions that can disturb air flow at a BSC or an animal isolator.
A Class I or II biological safety cabinet (see Clause 10.7) shall be provided if work with microorganisms transmissible by the respiratory route or work producing a significant risk from aerosol production is anticipated.
Installation and use, including the decontamination of the biological safety cabinet, shall be performed in accordance with the requirements of AS/NZS 2647.
Suitable containment equipment shall be provided to minimise personnel exposure to allergens where applicable.
61In addition to the work practices described in Clause 6.4.3 for Animal PC1 facilities, the following work practices shall be observed:
An Animal PC3 facility is suitable for work with infectious microorganisms in Risk Group 3 and incorporates equipment and practices for Animal PC1 facilities except Item 6.4.2(i) of Clause 6.4 and all equipment and practices for Animal PC2 facilities (Clause 6.5); however, additional requirements for construction, conditions of access, safety equipment and staff training apply.
When pathogenic microorganisms of Risk Group 3 are being used in association with small animals, primary containment devices such as BSCs or individually ventilated isolators fitted with HEPA exhaust filters should be used wherever practicable. Where primary animal contain merit devices cannot be used, the facility forms the primary containment measure.
NOTE: The design of a PC3 facility is complex and those planning its construction should seek specialized advice. See also Appendix G for examples of recommended layouts for PC3 facilities showing the design principles involved and Appendix H for airtightness considerations.
Similar requirements to those applying to Animal PC1 and Animal PC2 facilities also apply to Animal PC3 facilities, apart from the option to provide drinking water. In addition to construction requirements described for Animal PC1 and Animal PC2 facilities in Clauses 6.4.2 and 6.5.2, the following shall apply:
A ventilation system that establishes a negative pressure in the facility shall be provided so that there is a directional airflow into the working area. Where facilities have supply air systems, the supply air and exhaust air systems shall be interlocked, to ensure inward airflow at all times. The proper directional airflow into the facility shall be verified by airflow tests. The facility (including the airlock) shall be structurally designed to take account of the operation under negative pressures.
Failure of a single component, such as an exhaust fan or a supply fan, can result in extremely high positive or negative pressures in the facility. Alarms and failure mode operations of ventilation systems shall address this risk to ensure that interlocks operate rapidly to stop systems. The facility shall be constructed to withstand, without cracking or deterioration, the maximum positive and negative pressures that can be generated until failure mode safeguards operate. Automatic and manual failure mode sequences shall be independent of any automated control system that may, itself, be the primary cause of a failure situation.
All air that leaves the facility shall be exhausted in accordance with the requirements of this Clause.
Air may be recirculated within each facility. If air is recirculated, this shall be achieved utilizing internally-mounted airconditioning equipment such as fan coil units and split system airconditioning units. Any internally-mounted equipment shall be provided with removable panels as required to ensure the complete penetration of gas or vapour during room decontamination.
NOTES:
Ventilation equipment and outlets shall be located to minimize the disturbance to the open faces of Class I and Class II biological safety cabinets.
The facility ventilation shall incorporate the following features:
Access to voids surrounding the immediate perimeter of the facility and to the ventilation equipment that serves the facility shall be restricted to authorized persons. Items of equipment, ducts and access panels to contained sections of the ventilation system shall be marked with biohazard labels to minimize the risk of accidental exposures to air or to contaminated surfaces. The installation of services shall ensure proper access to equipment such as HEPA filters for maintenance and testing personnel and their equipment.
In addition to equipment specified for PC2 (Clause 6.5.4), a Class III Biological safety cabinet shall be provided where appropriate (see Clause 10.7.2).
In addition to requirements for Animal PC1 and Animal PC2 facilities (see Clauses 6.4 and 6.5), the following work practices shall apply:
See Clause 2.6.
68An Animal PC4 facility is suitable for work with infectious microorganisms. In Risk Group 4 and incorporates all equipment and practices for Animal PC1 (Clause 6.4), Animal PC2 (Clause 6.5) and Animal PC3 (Clause 6.6); however, additional requirements on conditions of access and egress, safety equipment and staff training apply.
An Animal PC4 facility may be one of two types—
When pathogenic microorganisms of Risk Group 3 or 4 are being used in association with small animals, primary containment measures such as BSCs or individually ventilated isolators fitted with HEPA exhaust filters shall be used. Where primary animal containment measures cannot be used, the facility forms the primary containment measure.
NOTE: The design of an Animal PC4 facility is complex and those planning its construction should seek specialized advice. See also Appendix G for examples of recommended layouts for PC4 facilities showing the design principles involved and Appendix H for airtightness considerations.
Animals may be kept in PC4 facilities in the following ways:
In addition to the design features and facilities specified for Animal PC1, Animal PC2 and Animal PC3, the following shall be provided:
For certain requirements, a specially designed suit area may be provided within the facility.
Personnel who enter this area shall wear a one-piece positive pressure suit that is ventilated by a life support system. If provided, positive pressure suit areas shall comply with the following additional requirements:
The facility ventilation shall comply with the following:
In addition to the requirements in Clause 6.7.4.1, the following ventilation system features shall be provided for a positive pressure suit area:
NOTE: A 25 Pa differential for each airlock is recommended.
For work with agents of Risk Group 4, either of the following shall be provided:
In addition to the work practices specified for Animal Physical Containment Levels 1 (Clause 6.4.3), 2 (Clause 6.5.4) and 3 (Clause 6.6.5), the following practices shall be observed:
In addition, the following work practices apply for positive pressure suit areas:
See Clause 2.6.
74Plant microorganisms are not usually directly hazardous to humans. They may, however, pose a significant hazard to the environment, agriculture and forestry. Plants infected with microorganisms classified into Plant Risk Groups 1 to 4 require corresponding physical containment level facilities. Selection of the level of containment required to prevent escape will depend on the biology of the organism and the impact that escape might have on the environment.
Hazards associated with plant facilities include propagules, such as seeds and pollen, tiny invertebrates and plant microorganisms. Compliance with microbiological, animal and invertebrate sections of this Standard shall be included where applicable.
Plant containment facilities are intended to prevent the escape of plants and seeds and limit the entry and escape of invertebrate vectors in order to prevent dissemination of plant infectious microorganisms.
This Section sets out requirements for four levels of plant physical containment (Plant PC) facilities for plants infected with microorganisms. The appropriate location, construction requirements and work practices are shown in Clause 7.2 for Plant PC1 facilities, while Clauses 7.3, 7.4 and 7.5 cover Plant PC2, Plant PC3 and Plant PC4 facilities respectively.
NOTE: This Section is not intended to cover the use of plant growth cabinets within laboratories.
The following standard of plant containment facilities and work practices (Plant PC1) is regarded as a suitable minimum for work with plants infected with plant microorganisms in Plant Risk Group 1. Plant PC1 facilities provide the most basic containment and include open fields and structures comprising greenhouses, screen houses and flexible film plastic structures.
Plant PC1 facilities have no special location requirements.
Plant PC1 facility structures shall comply with the following:
The work practices for Plant PC1 facilities shall be as follows:
NOTES:
The following level of plant containment facilities and work practices (Plant PC2) is regarded as a suitable minimum for work with plants infected with plant microorganisms in Plant Risk Group 2. Plant PC2 facilities incorporate all the appropriate requirements of
76Plant PC1 containment facilities as well as the additional requirements relating to location, construction and work practices specified in Clauses 7.3.2 to 7.3.4.
Plant PC2 facilities include permanent greenhouse structures with an anteroom or a corridor with self-closing doors to restrict access. Containment is achieved primarily through the creation of a physical barrier. Windows and ventilation inlets and outlets are screened and effective pest control procedures are in place.
The Plant PC2 facility should have a buffer zone of at least 3 m free of primary or alternative hosts that may be susceptible to infection from the plant microorganisms being used in the containment facility.
In addition to the construction requirements specified for Plant PC1 facilities in Clause 7.2.3, the following shall apply:
A Class I or II biological safety cabinet (see Clause 10.7) shall be provided if work producing a significant risk from aerosol production is anticipated.
Installation and use, including the decontamination of the biological safety cabinet, shall be performed in accordance with the requirements of AS/NZS 2647.
When infectious materials are used, a centrifuge fitted with either sealed rotors or sealed buckets shall be used. (See also Clause 10.3.)
In addition to the work practices described in Clause 7.2.4 for Plant PC1 facilities, the following work practices shall be observed:
The following standard of plant physical containment facilities and work practices (Plant PC3) is regarded as a suitable minimum for work with plants infected with plant microorganisms in Plant Risk Group 3. Plant PC3 facilities shall incorporate all the appropriate requirements of Plant PC1 and Plant PC2 facilities as well as those of Clauses 7.4.2 to 7.4.6.
79Plant PC3 facilities include permanent greenhouse structures with sealed windows and all ventilation inlets and outlets fitted with appropriate screens and filters to prevent ingress and egress of unwanted invertebrates. Containment is achieved primarily through good operational practices, the use of protective clothing and effective sanitation. Supporting containment is achieved by solid construction, negative pressure within the contained environment, and the use of HEPA exhaust air filters. Plant PC3 facilities are suitable for use with plants infected with exotic plant microorganisms that present a significant hazard to plants but have limited natural ability to be transmitted outside the facility.
NOTE: The design of a PC3 facility is complex and those planning its construction should seek specialized advice. See also Appendix G for examples of recommended layouts for PC3 facilities showing the design principles involved and Appendix H for airtightness considerations.
Plant PC3 facilities shall be protected against flooding and storm surges.
Structural design for wind loads shall take into account wind region maps (see AS/NZS 1170.2).
Where Plant PC3 facilities are proposed to be located in active seismic zones, the structural design shall take into account the potential for seismic damage.
In addition to the construction requirements specified for Plant PC1 and Plant PC2 facilities in Clauses 7.2.3 and 7.3.3, the following shall apply:
A ventilation system that establishes a negative pressure in the facility shall be provided so that there is a directional airflow into the working area. Where facilities have supply air systems, the supply air and exhaust air systems shall be interlocked, to ensure inward airflow at all times. The proper directional airflow into the facility shall be verified by airflow tests. The facility (including the airlock) shall be structurally designed to take account of the operation under negative pressures.
Failure of a single component, such as an exhaust fan or a supply fan, can result in extremely high positive or negative pressures in the facility. Alarms and failure mode operations of ventilation systems shall address this risk to ensure that interlocks operate rapidly to stop systems. The facility shall be constructed to withstand, without cracking or deterioration, the maximum positive and negative pressures that can be generated until failure mode safeguards operate. Automatic and manual failure mode sequences shall be independent of any automated control system that may, itself, be the primary cause of a failure situation.
82Air may be recirculated in plant facilities where there are no airborne risks to humans. All air leaving the facility shall be filtered with HEPA filters prior to recirculation. Air leaving the facility that is intended for recirculation shall firstly meet all applicable requirements of exhaust air in Items (d) to (h) inclusive prior to recirculation. Provision shall be made to isolate any unsealed sections of duct and equipment during gaseous decontamination and during post-gaseous decontamination purging.
Where air is recirculated within a plant facility, equipment used for this purpose, such as fan coil units and split system air conditioning units, shall be provided with removable panels as required to ensure the complete penetration of gas or vapour during room decontamination.
NOTES:
- Ventilation equipment should be located to ensure a flow of incoming air from the vicinity of the entry door towards the highest risk microbiological work areas.
- The quantity of outside air supplied to the facility should comply with relevant health quality standards or regulations (see AS 1668.2) and should dilute airborne contaminants.
Ventilation equipment and outlets shall be located to minimize the disturbance to the open faces of Class I and Class II biological safety cabinets.
The facility ventilation shall incorporate the following features:
Access to voids surrounding the immediate perimeter of the facility and to the ventilation equipment that serves the facility shall be restricted to authorized persons. Items of equipment, ducts and access panels to contained sections of the ventilation system shall be marked with biohazard labels to minimize the risk of accidental exposure to air or to contaminated surfaces. The installation of services shall ensure proper access to equipment such as HEPA filters for maintenance and testing personnel and their equipment.
In addition to work practices specified for Plant PC1 and Plant PC2 facilities, the following work practices shall apply:
The following standard of plant physical containment facilities and work practices (Plant PC4) is regarded as a suitable minimum for work with plants infected with plant microorganisms in Plant Risk Group 4. Plant PC4 facilities incorporate equipment and practices for Plant PC1, Plant PC2 and Plant PC3 (Clauses 7.2, 7.3 and 7.4); however, additional requirements on conditions of access and egress, safety equipment and staff training apply.
Plant PC4 facilities include permanent greenhouse structures with inward directional airflow to prevent microorganism escape. Plant PC4 facilities are suitable for use with plants infected with exotic plant microorganisms that present a significant hazard to plants and can readily spread outside the facility in the absence of a vector.
NOTE: The design of a Plant PC4 facility is complex and those planning its construction should seek specialized advice. See also Appendix G for recommended layouts for PC4 facilities showing the design principles involved and Appendix H for airtightness considerations.
In addition to the construction requirements specified for Plant PC1, Plant PC2 and Plant PC3, a Plant PC4 facility shall incorporate the following features:
The plant facility ventilation system shall comply with the following:
In addition to work practices specified for Plant PC1, Plant PC2 and Plant PC3, the following work practices shall apply:
See Clause 2.6.
89This Section provides requirements for containment of microorganisms associated with invertebrates. This necessarily includes containment requirements for the invertebrates themselves. The levels of containment defined in this Section relate to the risks posed by the microorganisms, not necessarily the risks posed by the invertebrate. Levels of invertebrate containment facilities correspond to the four Invertebrate Risk Groups. Selection of the level of containment required to prevent escape will depend on the nature of the invertebrate itself, any associated infectious microorganisms and its potential to act as a vector for human, animal or plant microorganisms. Any or all of these factors need to be considered to assess the impact on personnel and the environment.
NOTE: The principles and considerations relating to animal containment set out in Clauses 6.2 and 6.3 provide guidance on factors that may be relevant for some invertebrates.
Hazards associated with invertebrate facilities include escape of invertebrates and escape of microorganisms using vectors other than the invertebrates themselves. Compliance with microbiological, plant and animal sections of this Standard shall be included where applicable.
This Section sets out requirements for four levels of invertebrate physical containment (Invertebrate PC) for containing invertebrates. The appropriate location, construction requirements and operating procedures are shown in Clause 8.2 for minimum level Invertebrate PC1 facilities, while Clauses 8.3, 8.4 and 8.5 cover Invertebrate PC2, Invertebrate PC3 and Invertebrate PC4 facilities respectively.
Invertebrate PC3 is the minimum recommended level when dealing with invertebrates that may be carrying exotic microorganisms until the microorganism risk has been satisfactorily assessed. The invertebrates may be relocated to a lower or higher level of facility following analysis.
The following standard of invertebrate physical containment (Invertebrate PC1) facilities and work practices is regarded as a suitable minimum for work with Invertebrate Risk Group 1 microorganisms.
Invertebrate PC1 facilities have no special location requirements.
The Invertebrate PC1 facility shall comply with the following:
The work practices for Invertebrate PC1 facilities shall be as follows:
NOTES:
The following standard of invertebrate containment facilities and work practices is suitable for work with Invertebrate Risk Group 2 microorganisms. Invertebrate PC2 facilities incorporate all appropriate requirements of Invertebrate PC1 containment facilities as well as the additional requirements relating to location, construction and work practices specified in Clauses 8.3.2 to 8.3.5.
Invertebrate PC2 facilities include permanent structures with an anteroom with self-closing doors to restrict access. Containment is achieved primarily through the creation of a physical barrier. Windows and ventilation inlets and outlets are screened and effective pest control procedures are in place.
The potential impact of severe environmental events such as flood, fire, earthquake and high winds should be considered when selecting sites for Invertebrate PC2 facilities.
The Invertebrate PC2 facility should have a buffer zone free of primary or alternative hosts that may be susceptible to infection from microorganisms potentially being carried by the invertebrates in the containment facility. The extent of the buffer zone should be determined through consideration of the risks posed by a potential escape. Large distances or location of the facility in areas where there are no potential hosts may be appropriate.
In addition to the construction requirements specified for Invertebrate PC1 facilities in Clause 8.2.3, the following shall apply:
A Class I or II biological safety cabinet (see Clause 10.7) shall be provided if work producing a significant risk from aerosol production is anticipated.
Installation and use, including the decontamination of the biological safety cabinet, shall be performed in accordance with the requirements of AS/NZS 2647.
When infectious materials are used, a centrifuge fitted with either sealed rotors or sealed buckets shall be used. (See also Clause 10.3.)
93In addition to work, practices specified in Clause 8.2.4 for Invertebrate PC1, the following work practices shall be observed:
The following standard of invertebrate physical containment facilities and work practices (Invertebrate PC3) is regarded as a suitable minimum for work with Invertebrate Risk Group 3 microorganisms. Invertebrate PC3 facilities incorporate all the appropriate requirements of Invertebrate PC1 and Invertebrate PC2 facilities as well as those of Clauses 8.4.2, 8.4.3 and 8.4.4.
Invertebrate PC3 facilities include permanent structures with sealed windows and all ventilation inlets and outlets fitted with appropriate screens and filters to prevent ingress and egress of unwanted invertebrates. Containment is achieved primarily through good operational practices, the use of protective clothing and effective sanitation. Supporting containment is achieved by solid construction, negative pressure within the contained environment, and the use of HEPA exhaust air filters.
NOTE: The design of a PC3 facility is complex and those planning its construction should seek specialized advice. See also Appendix G for examples of recommended layouts for PC3 facilities showing the design principles involved and Appendix H for airtightness considerations.
Where located in areas that suffer from extreme climatic events (e.g. storms, cyclones), the design of the facility shall take these factors into account to minimize the risk of damage. Invertebrate PC3 facilities shall not be located in areas that are subject to flooding.
Structural design for wind loads shall take into account wind region maps (see AS/NZS 1170.2).
Where Invertebrate PC3 facilities are proposed to be located in active seismic zones, the structural design shall take into account the potential for seismic damage.
Invertebrate PC3 facilities shall not be located in areas that are geologically unstable or prone to land slippage.
In addition to the construction requirements specified for Invertebrate PC1 and Invertebrate PC2 facilities in Clauses 8.2.3 and 8.3.3, the following shall apply:
A ventilation system that establishes a negative pressure in the facility shall be provided so that there is a directional airflow into the working area. Where facilities have supply air systems, the supply air and exhaust air systems shall be interlocked, to ensure inward airflow at all times. The proper directional airflow into the facility shall be verified by airflow tests. The facility (including the airlock) shall be structurally designed to take account of the operation under negative pressures.
Failure of a single component, such as an exhaust fan or a supply fan, can result in extremely high positive or negative pressures in the facility. Alarms and failure mode operations of ventilation systems shall address this risk to ensure that interlocks operate rapidly to stop systems. The facility shall be constructed to withstand, without cracking or deterioration, the maximum positive and negative pressures that can be generated until failure mode safeguards operate. Automatic and manual failure mode sequences shall be independent of any automated control system that may, itself, be the primary cause of a failure situation.
Air may be recirculated in invertebrate facilities where there are no airborne risks to humans. All air leaving the facility shall be filtered with HEPA filters prior to recirculation. Air leaving the facility that is intended for recirculation shall firstly meet all applicable requirements of exhaust air in Items (d) to (h) inclusive prior to recirculation. Provision shall be made to isolate any unsealed sections of duct and equipment during gaseous decontamination and during post gaseous decontamination purging.
When air is recirculated within an invertebrate facility, equipment used for this purpose, such as fan coil units and split system airconditioning units, shall be provided with removable panels as required to ensure the complete penetration of gas or vapour during room decontamination.
NOTES:
Ventilation equipment and outlets shall be located to minimize the disturbance to the open faces of Class I and Class II biological safety cabinets.
The facility ventilation shall incorporate the following features:
Access to voids surrounding the immediate perimeter of the facility and to the ventilation equipment that serves the facility shall be restricted to authorized persons. Items of equipment, ducts and access panels to contained sections of the ventilation system shall be marked with biohazard labels to minimize the risk of accidental exposure to air or to contaminated surfaces. The installation of services shall ensure proper access to equipment such as HEPA filters for maintenance and testing personnel and their equipment.
In addition to equipment specified for PC2 (Clause 8.3.4), a Class III Biological safety cabinet shall be provided where appropriate (see Clause 10.7.2).
In addition to work practices specified for Invertebrate PC1 and Invertebrate PC2 facilities, the following work practices shall apply:
The following standard of invertebrate physical containment facilities and work practices (Invertebrate PC4) is regarded as a suitable minimum for work with Invertebrate Risk Group 4 microorganisms. Invertebrate PC4 facilities incorporate all equipment and practices for Invertebrate PC1, Invertebrate PC2 and Invertebrate PC3 (Clauses 8.2, 8.3 and 8.4); however, additional requirements on conditions of access and egress, safety equipment and staff training apply.
Invertebrate PC4 facilities include permanent structures with inward directional airflow to prevent the escape of invertebrates.
NOTE: The design of an Invertebrate PC4 facility is complex and those planning its construction should seek specialized advice. See also Appendix G for recommended layouts for PC4 facilities showing the design principles involved and Appendix H for airtightness considerations.
In addition to the construction requirements specified for Invertebrate PC1, Invertebrate PC2 and Invertebrate PC3 facilities, an Invertebrate PC4 facility shall incorporate the following features:
The invertebrate facility ventilation system shall comply with the following:
In addition to work practices specified for Invertebrate PC1, Invertebrate PC2 and Invertebrate PC3 facilities, the following work practices shall apply:
See Clause 2.6.
105The response for an inadvertent spill of biohazardous material in any type of containment facility (hereafter referred to in this Section as ‘spills’) will depend upon various factors such as the risk group of the microorganisms handled or if it is a human pathogen.
A risk assessment is an essential part of addressing microbiological hazards. This includes the determination of the correct response to any microbiological spill (see Clause 2.1.2).
Spills are categorized as follows:
The recommended response for each category is described in the clauses indicated. If unsure at any time of the correct procedure, ensure the safety of yourself and other workers, make the workplace as safe as possible and seek assistance.
In addition, procedures shall be developed and implemented for the following:
Planning for the control of a spill within the facility involves—
A spills clean-up team should be employed for cleaning up of spills where determined to be necessary by the risk assessment. This team shall have training in the correct response procedure for PC2 and PC3 containment facilities in the institution, the risk groups of the microorganisms handled and the correct use of any PPE, particularly respiratory protective equipment (RPE). Response procedures in PC4 facilities are best managed by the specialist scientific staff handling the infectious microorganisms.
106With large volume or highly hazardous microbiological spills, minimizing the spread of the contamination and preventing the production of aerosols are the guiding principles for protecting personnel. Leaving the area to avoid inhalation of infectious aerosols is the primary consideration. Allow at least 30 min for aerosols to settle or be removed by the air handling system before attempting to clean up the spill. Any contaminated PPE is left in the area where the spill occurred.
Refresher training for both staff and the spills clean-up team shall be done on a regular basis to ensure competence is maintained.
Emergency showers that are provided for chemical spills in laboratories are not suitable for decontamination of personnel who have been exposed to biological material. Such showers will spread the contamination and create more aerosols. This can be exacerbated by the lack of drains underneath most emergency showers.
Eyewash stations or hand-held drench hoses can be used when there is contamination of the eyes/face/mucous membranes with infectious material or chemicals, as waste can be contained in a sink. If the level of contamination necessitates a shower, then this can be taken in a regular shower after initial rinsing with a hand-held drench hose.
Clean-up materials and equipment should be kept at an appropriate location and should include the following:
This procedure applies to spills that occur inside all types of biological safety cabinets, irrespective of the level or type of containment facility.
Spills inside a biological safety cabinet are generally considered to be a lower hazard than those outside the cabinet as they are contained and aerosols are swept away by the cabinet air stream. Clean-up may be commenced immediately and may be done by the workers themselves.
Small spills, i.e. droplet-size spills or those up to 1 mL, may be treated easily by wiping with disinfectant-soaked absorbent material or flooding with a suitable disinfectant solution. Allow adequate time for the disinfectant to take effect.
The suggested procedure for a larger spill or breakage is as follows:
Spills outside biological safety cabinets may be of varying degrees of complexity, ranging from spills where a limited number of persons work to those occurring in high access areas such as corridors. All efforts should be directed towards minimizing the chance of a spill occurring. For PC2 and higher levels of containment, material containing microorganisms that is being moved in or between facilities or service areas shall be contained in secondary sealed, unbreakable containers.
Spills can involve amounts of material ranging from 1 mL or less, to more than 100 mL. The amount spilled, the physical characteristics of the material and how the spill occurred are important factors in determining the area of involvement.
When liquid is spilled, it is generally dispersed as three spill fractions:
The larger airborne particles settle rapidly, whereas the smaller particles can remain suspended in air for a considerable time and can be transported from the spill site by a ventilation system. In the event of a spill of liquid, it shall be assumed that an aerosol has been generated.
All staff shall learn the basic procedure for the control of microbiological spills. Decontamination procedures for spills of infectious material shall contain the contamination
108in the affected area. Spills in confined areas, especially cold-rooms, require special considerations e.g. the airconditioning system and air flow direction. General spills, such as from liquid cultures or culture plates, shall be treated with a suitable disinfectant.
NOTE: For spills at colder temperatures, longer contact times for disinfectants are required.
The treatment of microbiological spills in all levels and types of containment facilities shall be determined by the risk assessment.
After a spill has been cleaned up, an incident report shall be completed in accordance with any institutional requirements.
NOTE: An example incident report form is given in Appendix B.
Spills inside BSCs should be dealt with as described in Clause 9.3.
In spills external to BSCs, low hazard (as determined by the risk assessment) infectious material that is spilled without generating significant aerosol, and does not contain a human pathogen spread by the respiratory route, should be cleaned up with a paper towel or other absorbent material soaked with an effective chemical disinfectant.
The response should be as follows:
Spills inside BSCs should be dealt with as described in Clause 9.3.
A spill external to a BSC of a large volume of high risk (as determined by the risk assessment) infectious material with the generation of aerosols will require evacuation of the area and clean-up by a trained spills clean-up team. This team shall wear protective clothing and RPE if the spill is hazardous to humans by the respiratory route.
Once a spill of this type has occurred, the area shall be evacuated immediately and sufficient time allowed (generally 30 min) for aerosol particles to be dispersed before contaminated surfaces are disinfected.
NOTE: Although in certain circumstances respirators with P2 filters can provide adequate respiratory protection, the higher protection offered by HEPA filters with a full face respirator is recommended for spill clean-up operations. Goggles should be worn where full face respirators are not used.
The response for the worker should be as follows:
The response for the spills clean-up team should be as follows:
Spills inside BSCs should be dealt with as described in Clause 9.3.
Spills in PC3 facilities that occur outside BSCs can be dealt with either by the workers themselves, as they have experience with the microorganisms handled, or by a specialist trained spills clean-up team.
NOTE: The clean-up team may require vaccinations to enable entry into the PC3 facility.
The response will vary with the design of the facility but should follow the basic procedures detailed in this Clause.
The response for the worker should be as follows:
The response for the spills clean-up team (who may be the workers themselves) should be as follows:
NOTES:
Spills inside BSCs are less hazardous and should be dealt with as described in Clause 9.3.
111Spills that occur in PC4 facilities outside BSCs where no positive pressure suits are worn, are particularly hazardous due to the nature of Risk Group 4 microorganisms. It is imperative that exposure to aerosols is minimized.
The clean-up procedure should be carried out by the specialist scientists working in the PC4 facility, who have been trained to deal with this situation. The procedure should be as follows:
Spills inside BSCs should be dealt with as described in Clause 9.3.
Spills that occur in PC4 facilities outside BSCs where positive pressure suits are worn, are less hazardous than those where no suits are worn (see Clause 9.4.5), as the workers are fully protected against any aerosols.
Clean-up of the spill may begin immediately and should be carried out by the specialist scientists working in the PC4 facility who have been trained to deal with this situation, following similar principles to those described in Clause 9.4.5.
Complete an incident report form after exiting the PC4 facility.
Where a spill or leak is detected within a centrifuge, the procedure will depend upon the risk group of the agent involved (see Clause 9.1) as well as the construction of the equipment. The clean-up procedure should be as follows:
112Many media components, chemicals and reagents used in the microbiological laboratory are hazardous to health, however, the effects of some of these have not been fully characterized. The relevant material safety data sheet (MSDS) should be consulted for every chemical used to ensure that appropriate safety precautions are implemented to minimize the risk to health. AS/NZS 2243.2 and AS/NZS 2243.10 should be referred to for the safe use and storage of chemicals in laboratories and AS/NZS 2243.8 and AS/NZS 2243.9 should be referred to for the use of fume cupboards and recirculating fume cabinets respectively.
Fume cupboards and recirculating fume cabinets shall not be used when working with infectious materials. Conversely, fume cupboards, recirculating fume cabinets or local exhaust ventilation shall be used when determined appropriate by a risk assessment for work with toxic, odoriferous, volatile or corrosive substances.
Personal protective equipment and clothing can act as a barrier to minimize the risk of exposure to aerosols, splashes and accidental inoculation and shall be worn when working in microbiological containment facilities. The equipment shall be selected to suit the type of work being performed and the potential risk of exposure. AS/NZS 2243.1 should be consulted for detailed information on the types and use of PPE.
All PPE shall be removed and hands decontaminated prior to leaving the laboratory or containment facility.
Long-sleeved, back-opening gowns or coveralls should be used as they give better protection than laboratory coats. Where necessary to give further protection against spillage of chemicals or biological materials such as blood or culture fluids, aprons should be worn over gowns or laboratory coats.
Closed footwear shall be worn, i.e. footwear that covers the toes and heels, unless lesser requirements can be justified by a risk assessment. Where specific safety footwear is required for a particular hazard, it shall be selected in accordance with AS/NZS 2210.
Protective eyewear shall be worn unless a documented risk assessment can justify a lesser requirement. The choice of equipment to protect the eyes and face from splashes and impacting objects is dependent on the activity performed. Prescription or plain eye protectors are manufactured using shatterproof material and either curved or fitted with side shields. Goggles or overglasses may be worn over normal prescription spectacles. Contact lenses do not provide protection against laboratory hazards. Face shields are made of shatterproof plastic, fit over the face and are held in place by head straps or caps. Units with chin guards are preferable. AS/NZS 1336, AS/NZS 1337 and the AS/NZS 1338 series shall be consulted when choosing the type of eye protection to be used.
114Microbiological work should be planned to limit the reliance on respiratory protective equipment (RPE). Most laboratory work with microorganisms transmissible to humans by the respiratory route is conducted in containment equipment such as a BSC. Respiratory protective equipment shall be used when carrying out highly hazardous procedures, e.g. when cleaning up a spill of material containing microorganisms transmissible by the aerosol route and handling animals infected with zoonotic agents transmissible by the respiratory route. Where possible, animals infected with zoonotic agents transmissible to humans by the respiratory route are housed in ventilated cages fitted with exhaust HEPA filters.
The various types of RPE available afford different levels of respiratory protection. To protect against contaminants in the atmosphere, exposure standards have been determined for particulates and specific chemicals. AS/NZS 1715 provides information on the types of RPE, types of filters and the selection of appropriate RPE for a particular situation. AS/NZS 1715 also describes the two ways of providing personal respiratory protection, i.e. purifying the air that a person breathes and supplying the person with respirable air.
Protection factors (PFs), which are a measure of the degree of protection afforded by a particular respirator, are used to determine the reduction in exposure that a particular respirator type can be expected to provide. These can be used to assist in the selection of RPE. These values have been determined mainly for particulates and chemicals (see References 1.10, 1.11 and 1.12), but similar values are obtained (see Table 10.1) when RPE is tested against aerosolized bacteria and fungal spores (see Reference 1.13). The determination of precise PFs for infectious agents is difficult because air concentrations of infectious agents are often impossible to measure and infectious doses (exposure limits) are not available for most diseases. The lack of exposure standards for microorganisms means that the concept of minimum protection factors (MPF), as described in AS/NZS 1715. cannot be easily applied. Therefore, although imprecise, qualitative professional judgments are needed to evaluate particular exposure hazards, the level of protection required, the convenience of a device in a particular circumstance, the type of work to be done and other relevant factors. A minimum protection factor is defined in AS/NZS 1715 as the level of respiratory protection that an item of properly functioning RPE or class of RPE would be expected to provide to properly fitted and trained users in the workplace, when used in accordance with the manufacturer’s information and instruction. The MPF takes into account all expected sources of facepiece penetration (e.g. face seal protection, valve leakage). (By way of contrast, some current overseas publications quote higher protection factors for respirator classes by not taking all these factors into account.)
| MPF* | Respirator type | Comment |
|---|---|---|
| 10 | Halt-facepiece (P2 or N95) | Respirator needs to be fit tested to the individual |
| 50 | Powered air purifying respirator (PAPR) with half-facepiece | Devices covering half-face provide lower levels of protection |
| 50 | Full-facepiece respirator with P3 or HEPA filter | Respirator needs to be fit tested to the individual |
| 100 | PAPR with P3 or HEPA filter and head-covering hood | Considered to provide high protection. Fit testing to the individual not required |
| 10 000 | Self-contained breathing apparatus (SCBA) with positive pressure demand | Not practical for most microbiological applications |
| * Minimum protection factor that could be assigned to the respirator type | ||
Air-purifying RPE is available with interchangeable filters for protection against gases, vapours, particulates and microorganisms. SCBA and supplied air respirators provide the highest level of protection but are impractical in most microbiological applications. RPE employing a HEPA filter (P3 filter) provides the best practical respiratory protection against aerosolized microorganisms. Air-purifying RPE can be powered (where air is drawn through the filter by means of a fan) or non-powered (where air is drawn through the filter by wearer inhalation). In either case, the RPE may be in the form of a half facepiece (includes disposable type), a full facepiece or a head covering. The performance of full face and half face respirators is critically dependent on fit testing as described in AS/NZS 1715.
The term ‘face masks’ is used to describe masks designed for use in health care, such as in operating rooms, medical and dental procedures. These types of masks are covered in AS 4381. They are not for use where an additional degree of respiratory protection is required from the risk of airborne transmission of infection, and they do not meet the requirements for RPE specified in AS/NZS 1715.
Contamination of hands may occur when laboratory procedures are performed. Hands are also vulnerable to ‘sharps’ injuries. Disposable latex, nitrile or vinyl surgical-type gloves are used widely for general laboratory work, particularly when blood and body fluids are being handled or to protect laboratory materials from human contamination.
After handling infectious materials, working in a biological safety cabinet and before leaving the laboratory, gloves shall be removed and hands decontaminated. Used gloves shall be discarded with infected laboratory waste.
Allergic reactions such as dermatitis and immediate hypersensitivity have been reported in laboratory and other workers wearing latex gloves (powdered and non-powdered). Alternatives such as nitrile or vinyl gloves should be made available.
Heat-insulating gloves should be worn when conducting procedures involving liquid nitrogen, sterilizers and microwave ovens.
Stainless steel mesh gloves should be worn when there is a potential exposure to cuts from sharp instruments, e.g. during post-mortem examinations.
Further information on occupational protective gloves is available in the AS/NZS 2161 series.
While centrifuges are essential pieces of laboratory equipment, equipment failure, such as the breakage of centrifuge tubes or buckets, can pose a hazard to operators, to other staff, to the environment and to other experimental work through the release of contaminated aerosols. To reduce the potential for aerosol release, aerosol-tight, sealed buckets or sealed rotors should be used.
NOTE: The life of some types of seals or tubes can be shortened by repeated steam sterilization (consult the manufacturer’s guidelines).
The following measures shall be implemented when using centrifuges:
The following procedures shall be implemented:
Where sealed-bucket or sealed-rotor centrifuges are not available, the following precautions shall be taken:
Laboratories using a number of large, medium-speed and high-speed centrifuges often install them in a dedicated centrifuge room. To allow for the possibility of a rotor failure or leakage with a resultant production of an aerosol, the centrifuge room ventilation shall be treated according to the requirements of its physical containment level.
Freeze-drying is an operation that is potentially hazardous, both to susceptible hosts and to the laboratory environment. Freeze-drying shall be carried out in containment levels appropriate to the risk group of the microorganism being handled (see Section 4). To minimize the risks, the following points shall be observed:
Liquid nitrogen is commonly used for storing and transporting materials and cultures of microorganisms at low temperature. The very low temperature of the liquid, –196°C, will cause injury similar to high temperature thermal burns following very brief contact with body surfaces. The eyes are especially vulnerable to exposure. The following precautions shall be taken when handling liquid nitrogen and when adding or removing materials from low temperature storage:
Pressure steam sterilizers are used in facilities both for sterilization of media and equipment required for the culture of microorganisms, and for decontamination of discarded cultures and waste materials. Pressure steam sterilizers operate at high pressures and temperatures, and appropriate measures shall be taken for personnel safety. Relevant regulations for pressure vessels shall be consulted for information about regular certification of the sterilizer.
Persons using a pressure steam sterilizer shall be trained so they understand that correct loading of the sterilizer is essential to ensure sterilization or decontamination of the load. Operators shall be trained so they understand the hazards associated with heat, steam and pressure. Operators shall be provided with protective clothing, including heat-insulating gloves, for use when loading and unloading sterilizers. Use of a face shield is recommended when unloading the sterilizer.
Areas for the temporary holding of material awaiting sterilization shall provide appropriate storage conditions and adequate protection from unauthorized access and vermin. Such areas should also have provision for the separate storage of infectious waste in impermeable plastic bags or lidded containers. Bags should be opened prior to commencement of the sterilization cycle.
Sterilization facilities shall be equipped with local exhaust ventilation with air capture vents or extraction systems for the removal of heat, steam and odours. Wire racks or perforated metal shelving shall be provided in the vicinity of heat sterilizers for the cooling of sterilized materials and loads. Adequate space shall be provided for the movement of large loads and trolleys.
Appropriate chemical disinfectants shall be provided for spills and leaks. Easy access to hand washing facilities, safety showers and eyewash facilities shall also be provided.
For efficient sterilization, all air shall be removed from the load and from the chamber of the sterilizer. This can be achieved by—
Downward-displacement cycles are used for the sterilization of articles, culture media and fluids. Small articles such as test tubes or bottles should be packed in open mesh baskets or similar containers allowing easy displacement of air. Screw caps should be loosened. Large containers such as buckets, trap air in downward-displacement cycles and should not be used to hold small articles for sterilization. If such large containers are to be sterilized when empty, they shall be placed on their sides in the chamber. Admission of steam at a controlled rate may be necessary to prevent damage to glassware. When using autoclave bags, extra water may be carefully added to the opened bag to assist in reaching the correct temperature for decontamination.
The timing of the sterilization stage of the cycle commences when the set temperature is recorded by the thermocouples in the drain line and in the densest part of the materials to be sterilized or decontaminated. Constituents of the load may not have reached this temperature and additional time for heating should be allowed especially where large containers of liquids or solids are to be sterilized. On the other hand, materials that may be damaged by excessive heat over an extended period should not form part of a load containing large volumes of liquid.
119Procedures used shall address the dangers of removing containers of fluid from the hot sterilizer chamber. Sufficient time should be allowed for cooling before they are handled. Persons using the sterilizer shall ensure that the sterilization cycle is complete and the pressure has returned to zero before attempting to open the sterilizer door. Care should be taken when removing large containers of liquid after completion of sterilization, as sudden changes in pressure and temperature may occur and they may break or boil over when moved. Before removal of the load, the sterilizer door should be partly opened and sufficient time allowed for the load to cool. Avoid inhaling harmful vapours when opening a sterilizer if the load contains chemicals, e.g. biochemical test reagents such as amyl alcohol (l -pentanol) from the indole test.
AS 2192 should be consulted for requirements for downward-displacement steam sterilizers.
Penetration of steam into the load is inhibited in a downward-displacement cycle if air is trapped among cavities or in gaps in porous materials. The attainment of effective sterilizing temperatures at such sites is consequently delayed, or even prevented. Porous loads such as clothing should therefore be processed in a sterilizer fitted with a pump for air removal in a pre-vacuum stage of the cycle. If drying of the load is required, the same pump is also used in a post-vacuum stage, after sterilization. Pre-vacuum sterilizers can also be used to sterilize large empty containers that would trap air in downward-displacement cycles. Where there is a risk of microbial contamination in the evacuated chamber air, a 0.2 μm hydrophobic membrane type filter shall be fitted between the chamber and the vacuum pump and periodically maintained.
AS 1410 should be consulted for requirements for pre-vacuum steam sterilizers.
Sufficient penetration time should be allowed for all parts of the load to reach the desired temperature. Minimum holding times after attainment of temperature shall be—
Some visual indicators, such as sensitive papers or tapes, give only an indication that the sterilizer load has reached a specified temperature and do not give an indication of how long the load has been exposed to that temperature. Such visual indicators may be used as a check that materials have been processed, but shall not be used to monitor the efficacy of the sterilization procedure. Other chemical indicators progressively change colour with the time exposed at specified temperatures, and their use is recommended as they give an immediate indication of the efficacy of treatment.
Biological indicators should be used at regular intervals (e.g. monthly) to monitor the microbial killing power of the sterilization process. They shall be placed in several positions in a load, including those least likely to attain accepted sterilization parameters. Bacterial enzyme indicators may be used instead of biological indicators for the monitoring of sterilization cycles. These indicators are designed so that the loss of enzyme activity parallels the loss of spore viability. Their advantage is that enzyme inactivation can be easily and rapidly determined, e.g. within minutes or hours, by the addition of a substrate and observation for absence of a coloured or fluorescent end-point. In contrast, biological indicators require incubation for growth for periods of days.
The Bowie-Dick test (see AS 1410) is designed for the daily monitoring of air removal from standard towel packs sterilized in pre-vacuum sterilizers, and is not suitable for downward-displacement sterilizers.
120Sterilizer cycles should be validated. Validation is achieved by demonstrating that pre-determined physical and biological parameters can be met. Physical parameter validation involves demonstration that the pre-determined temperature can be reached in the coolest part of the sterilizer and the densest part of the load. This may be achieved by the use of thermocouples or resistance thermometers to demonstrate that the sterilization temperature selected is achieved. All gauges including temperature, pressure and time shall be calibrated. Calibration of gauges shall be performed by a trained competent person using measuring equipment that has a current certificate of calibration issued by a body with third-party accreditation for conducting such calibrations. Calibration should be performed on a regular basis and, as a minimum, annually. Biological validation involves successful demonstration of biological lethality through the placement of biologic/enzymatic indicators in the coolest part of the sterilizer (usually the drain) and in the densest part of a load. Generally, biologic/enzymatic indicators should be placed adjacent to the temperature sensors. Table 10.2 lists commonly-used biological indicators and has been based on information in Reference 1.14.
A logbook recording details of sterilizer load and cycle should be maintained. The chart records of temperature and duration of sterilization cycles should be assessed and checked regularly by the safety officer to ensure that the sterilizer cycle is maintained within calibration specifications.
Pressure relief valves shall discharge in a safe place outside and away from the containment structure because of the potential for a saturated atmosphere to damage the integrity of the containment facility.
The inner door shall automatically interlock with the outer door in such a manner that the outer door can be opened only after the sterilization cycle has been completed. In addition, all displaced or evacuated air, steam and liquid shall be regarded as potentially contaminated and shall be filtered or heat treated appropriately. Pressure sensing instruments shall be protected by filters that can be steam sterilized. All potentially contaminated pipework that is not steam sterilized shall be arranged to facilitate chemical decontamination.
NOTE: It should be noted that the liquid in liquid ring vacuum pumps is potentially contaminated and would require heat or chemical decontamination unless the evacuated air or gases have been filtered through an appropriate membrane filter.
Sterilizers used in PC3 and PC4 facilities shall be fitted with sealed bonnet pressure relief valves and be preceded with appropriately rated bursting discs. The interspace shall be monitored for pressure rise.
| Process | Species | Incubation temperature |
|---|---|---|
| Steam under pressure | Geobacillus stearothermophilus | 56°C Rapid enzyme BI (60°C) |
| Dry heat | Bacillus atrophaeus | 37°C |
| Ethylene oxide | Bacillus atrophaeus | 37°C |
| Subatmospheric steam and formaldehyde |
Geobacillus stearothermophilus | 56°C |
Two classes of biological safety cabinets are in common use, Class I and Class II, see Clause 1.4.7.
To enhance containment of hazardous materials in Class I or Class II cabinets, both AS 2252.1 and AS 2252.2 require that all potentially contaminated zones under positive air pressure are surrounded by zones of negative air pressure relative to the facility. Cabinets without this design feature may not provide the same degree of safety for the user and the environment.
In addition, Class II biological safety cabinets meeting AS 2252.2 are required to pass an air barrier containment test. This test is a direct determination of the effectiveness of containment by the air barrier and is part of the certification done regularly in the facility as required by AS/NZS 2647.
Class I and Class II cabinets, complying with AS 2252.1 and AS 2252.2 respectively, offer an equivalent degree of protection to the operator. Class I and Class II cabinets are designed to be freestanding units, and shall not be connected directly to ducting that vents to the atmosphere, as wind effects may interfere with containment. Exhaust air from Class I or Class II biological safety cabinets, which has been passed through a HEPA filter, may be discharged either into the facility or exhausted through the building exhaust system. When the building exhaust system is used, the connections shall be made in a manner that avoids any interference with the air balance of the cabinets (see AS/NZS 2647). Information on the installation and use of biological safety cabinets is provided in AS/NZS 2647.
All cabinets shall be checked for containment efficiency and safety before initial use, after any modification including change of HEPA filters, after relocation and on an annual basis. The use of Bunsen burners in Class II cabinets is not recommended as it disrupts the laminar flow and the barrier air. An alternative means, such as disposable implements or electrical heating, is preferred.
Cabinets shall be decontaminated with formaldehyde gas or an equivalent decontaminant before testing when they have been used for handling Risk Group 2, 3 or 4 microorganisms. Penetration of the decontaminant throughout all sections of the cabinet is essential.
When a cabinet is used for handling Risk Group 1 microorganisms or uninfected cell lines, a thorough wipe-down of all work area surfaces, including the inner surface of the viewing window, with a detergent/disinfectant cleaner shall be done before servicing and testing.
Clean workstations (laminar flow clean benches) conforming to AS 1386.5 do not provide operator protection as do biological safety cabinets. Clean workstations provide HEPA filtered air to protect the work in a vertical (downflow) direction or in a horizontal (crossflow) direction. Part or all of this air moves towards the operator. These workstations shall not be used when handling microorganisms of Risk Groups 2, 3 or 4 or hazardous materials.
122NOTE: Aspects of location, use, decontamination and outline of testing of biological safety cabinets are covered in an audiovisual presentation prepared by the WHO Collaborating Centre for Biosafety in Microbiology at the Victorian Infectious Diseases Reference Laboratory. The presentation is available from the publications list on the Victorian Infectious Diseases Reference Laboratory web site, www.vidrl.org.au. It includes the following parts:
- Part 1: Types of cabinets and their proper location;
- Part 2: Using the Class II cabinet;
- Part 3: Decontamination and testing of cabinets; and
- Part 4: Summary.
Class I and II biological safety cabinets shall not be used for the handling of infectious materials which also contain volatile hazardous chemicals, unless the exhaust air from the cabinets is removed via the building exhaust system and is not discharged into the room or specialist advice is sought on how to also capture the volatile hazardous chemicals.
Fume cupboards shall not be used when working with infectious materials.
A Class III BSC is a self-contained, totally enclosed chamber incorporating an isolator envelope and gloves attached to sleeves, for the performance of laboratory work with infectious material and for housing infected animals. Class III BSCs include flexible film isolators and some powder containment chambers used for handling powders suspected of being bioterrorist agents.
NOTES:
- The operator works in gloves attached to sleeves which are part of the BSC, or in gauntlets attached to the BSC envelope.
- Class III BSCs operate at a pressure below that of the room in which they are located. The entry and escape of air-borne particles is prevented by a HEPA filtered inlet and exhaust air system.
- Material is introduced and removed from the Class III BSC through supply and sample ports without compromising microbiological security.
- The flexible film isolator envelope is constructed of plastic film which is flexible, puncture and tear-resistant and optically clear and attached to a rigid supporting frame.
Laminar flow cytotoxic drug safety cabinets are suitable for work with materials containing prions. These cabinets, in contrast to laminar flow biological safety cabinets (Class II BSC), provide protection for cabinet maintenance staff in addition to protection of the environment, the material being handled and the operator. See AS 2567 and AS 2639.
As it is not possible to gaseously inactivate material containing prions, the design of the laminar flow cytotoxic drug safety cabinet enables this material to be captured in an exhaust filter located under the work floor. This arrangement prevents the contamination of the airflow paths within the cabinet. The procedure for sealing and safe removal of the cabinet exhaust HEPA filter is described in AS 2639. The labelling of the encapsulated filter shall be ‘Caution: Prion contaminated waste. Dispose by high temperature incineration only.’ Packaging shall also include the biological hazard symbol.
HEPA filters for containment facilities shall be either of the following:
HEPA filters for containment facilities shall be mounted in gastight housing(s) located as close as possible to the containment facility to minimize the length of potentially
123contaminated ductwork. The interconnecting ductwork between the containment room and the HEPA filter housing shall also be of gastight construction.
The design of the filter housing shall facilitate the testing of the integrity of the HEPA filter element and mounting, and the periodic gaseous decontamination of the filter element and associated mounting surfaces independently of the gaseous decontamination of the facility.
Housings shall be placed in fully accessible locations outside the facility with clear access to facilitate filter integrity testing, physical handling of filter elements and operation of isolating valves. Installations in false ceiling spaces should be avoided.
Filter housings shall incorporate the following features:
NOTE: Recommendations for airtightness of HEPA filter housings and the duct connections between the facility and the housings can be found in the section on the air handling system in Agriculture and Agri-Food Canada Veterinary Biologies Guideline 4.7E, Containment Standards for Veterinary Facilities, available from the Canadian Food Inspection Agency web site at www.inspection.gc.ca/english/sci/lab/convet/convete.shtml.
HEPA filters shall be tested in accordance with AS 1807.6 or AS 1807.7, as applicable, at least annually. Prior to testing, the HEPA filter shall be decontaminated. See AS/NZS 2647 for information on gaseous decontamination of biological safety cabinets and their HEPA filters.
124The facility’s physical containment level shall be considered when setting out cleaning arrangements and services. Dedicated cleaning equipment shall be provided for PC3 and PC4 facilities. Such equipment shall be stored within the containment facility.
Clean surroundings facilitate clean work. Staff shall clean and tidy work benches and shelves as they work, and provide a complete clean-up at the end of the working day.
Work areas shall be kept free from physical hazards that might cause spillages or breakages. Items for sterilization shall be regularly collected. This collection shall be independent of the regular collection of tin contaminated waste.
Special instructions for the cleaning of microbiological facilities shall be issued (particularly to cleaning contractors). Cleaning shall be carried out by trained personnel engaged for this purpose. Where cleaning contractors are used, their work should be confined to floor and window cleaning and removal of clearly marked uncontaminated waste. Only laboratory or facility staff shall handle infectious materials.
Apparatus such as centrifuges, water baths, incubators, refrigerators, deep freeze cabinets and liquid nitrogen storage vessels shall be cleaned and, if necessary, decontaminated at regular intervals and before being sent for repair or disposal.
Walls shall be cleaned periodically, or when visibly dirty, by washing with a detergent solution. Unnecessary or too-vigorous cleaning is not recommended, as it may cause damage to paint surfaces and provide a surface that is difficult to decontaminate.
Open shelves collect dust and shall be cleaned routinely. Frequently used reagent bottles and books collect little dust, but those seldom used may become dusty, and should be stored in closed cupboards.
The time of the day allocated for floor cleaning shall be specified. General floor cleaning should not be done during normal working hours, as it may produce dust and aerosols which contaminate work. The various floor cleaning methods are as follows:
Contaminated materials and non-contaminated waste shall be collected in segregated containers, clearly identified according to the following categories:
NOTE: See also AS/NZS 3816 and NZS 4304 for clinical and healthcare waste and AS/NZS 2243.1 for other types of waste.
All types of contaminated or potentially contaminated wastes, both liquid and solid, shall be decontaminated by one or more of the following methods:
After decontamination or chemical treatment, waste shall be disposed of in accordance with relevant authority requirements.
NOTE: Section 7 should also be consulted for specific requirements for plant materials.
Waste processes that include heat, pressure, chemicals, or a combination of these parameters, to achieve decontamination shall be periodically validated to ensure ongoing efficacy.
This validation shall form part of the waste decontamination risk assessment. The following should be carried out on a regular basis:
The following measures shall be adopted:
NOTE: See also Clause 10.6 for correct use of pressure steam sterilizers.
Following decontamination with appropriate disinfectant (see Appendix F), waste shall be disposed of in accordance with AS/NZS 2243.2 and relevant authority requirements.
Incineration of contaminated or potentially contaminated materials, e.g. sharps containers, shall be done using a high-temperature, high efficiency EPA-approved (Australia) or regional council-approved (New Zealand) incineration facility. Transport of materials by surface to such incinerators in Australia shall be done in approved packaging and according to the requirements of AS 4834 (see also Clause 13.4.2 (c)). Transport by air shall be according to IATA requirements (see Section 13).
NOTE: EPA-approved incineration for cytotoxic drugs exceeds the requirements for microbial destruction (see also Reference 1.17).
In some States in Australia, contaminated or potentially contaminated waste may be decontaminated and disposed of by approved methods other than those described in Clauses 12.2.3 to 12.2.5. Relevant authorities shall be consulted for advice.
Current recommendations for the sterilization of articles or specimens that could be contaminated by prions are 18 min at 134°C to 138°C in a pre-vacuum pressure steam
128sterilizer (UK) or 1 h at 132°C in a downward displacement pressure steam sterilizer (USA). The recommended chemical disinfectant for effective decontamination of prions is 20 000 p.p.m. available chlorine for 1 h with sodium hypochlorite as the chlorine releasing agent.
Pressure steam sterilizer/chemical methods for decontaminating heat-resistant instruments are either—
If the materials have already been fixed in formalin, then these steam sterilizing processes will not decontaminate them. The most effective chemical treatment for decontaminating formalin-fixed tissue is 96% formic acid for 1 h. For destruction of formalin-fixed tissues, steam sterilization in 1 M sodium hydroxide at 121°C for 1 h is effective for disposal. (See Clauses 1.15 and 1.16).
Radioactive waste should not be pressure steam sterilized.
In Australia, radioactive waste shall be treated in accordance with AS 2243.4 and Commonwealth, State or Territory requirements. The method used for the treatment and disposal of radioactive infectious waste depends on the isotope being used and whether the waste is liquid or solid. Seek advice from the Radiation Protection Officer.
In New Zealand, radioactive waste shall be treated and disposed in accordance with the requirements of the National Radiation Laboratory Code of safe practice for the use of unsealed radioactive materials, NRL C1.
Chemical waste shall be disposed of in accordance with AS/NZS 2243.2 and Government requirements where applicable.
General uncontaminated waste, e.g. paper towels from PC1 and PC2 facilities, may be disposed of in the same manner as household waste. All waste from PC3 and PC4 facilities shall be treated as potentially contaminated.
129International and national procedures have been established for the safe transport of biological materials by air, rail and road. Different packaging and transport arrangements apply depending on whether the materials are infectious substances, biological products, cultures, genetically modified microorganisms, medical or clinical wastes or exempt substances. It is the responsibility of the sender to ensure compliance with all packaging and transport regulations.
NOTE: In Australia, Item 92.120 of the Civil Aviation Safety Regulations specifies required training for packing dangerous goods for transport by air. All persons who pack dangerous goods for transport by air (including enclosing the goods in packaging, marking or labelling the consignment or preparing a shipper’s declaration) are required to successfully complete a course approved by the Civil Aviation Safety Authority, Australia.
This Section summarises the requirements of the various regulatory bodies and is based on United Nations Recommendations on the Transport of Dangerous Goods. Model Regulations, which are adopted by the International Air Transportation Association (IATA) and AS 4834.
Facilities should be provided for after-hours delivery of samples. After hours staff shall be warned of any hazards.
Precautions taken during unpacking procedures shall be consistent with the risk posed by the stated contents of the package.
If contaminated waste is to be removed from a facility, the relevant agricultural, veterinary, quarantine and local public health regulations shall be followed. (See also Section 12.)
The transport of biological materials is regulated by the following documents:
The IATA Dangerous Goods Regulations are the most comprehensive regulations and, in general, include the requirements of the other regulations. These regulations define the requirements for certification, packing instructions, the maximum quantities that can be transported by cargo or passenger aircraft, the external labelling requirements (including the identifying UN number), and the details to be included in the attached Shippers Declaration for Dangerous Goods. AS 4834 covers packaging for surface transport of biological material that may cause disease in humans, animals and plants in Australia.
The following definitions align with the UN Model Regulations and are used in this Section:
Infectious substances are substances which are known or are reasonably expected to contain pathogens. Pathogens are defined as microorganisms (including bacteria, viruses, rickettsiae, parasites, fungi) and other agents such as prions, which can cause disease in humans or animals.
Biological products are those products derived from living organisms which are manufactured and distributed in accordance with the requirements of appropriate national authorities, which may have special licensing requirements, and are used either for prevention, treatment, or diagnosis of disease in humans or animals, or for development, experimental or investigational purposes.
Cultures are the result of a process by which pathogens are intentionally propagated. This definition does not include patient specimens.
Patient specimens are those collected directly from humans or animals, being transported for purposes such as research, diagnosis, investigational activities, disease treatment and prevention.
Medical or clinical wastes are wastes derived from the medical treatment of animals or humans or from bioresearch.
Genetically modified mierooganisms are microorganisms in which genetic material has been purposely altered through genetic engineering in a way that does not occur naturally.
Clauses 13.4.2 to 13.4.5 provide details of the classifications that apply within the different types of biological materials and the corresponding packing requirements.
Figure 2 provides a flow chart summarizing the IATA, UN and AS 4834 requirements for the transport of biological materials by air, sea and land.
131NOTE: The IATA Dangerous Goods Regulations are updated annually with occasional amendments. The categories and flow chart are based on the 2005 edition. As requirements are likely to vary, the current edition and any amendments should be consulted.
Infectious substances shall be classified as Division 6.2 dangerous goods and assigned the appropriate UN number, UN 2814, UN 2900, UN 3291 or UN 3373 using the following categories and classification criteria:
An infectious substance which is transported in a form that, when exposure to it occurs, is capable of causing permanent disability, life-threatening or fatal disease in otherwise healthy humans or animals. Table 13.1 provides a list of indicative examples of substances that meet these criteria.
NOTE: An exposure occurs when an infectious substance is released outside its protective packaging, resulting in physical contact with humans or animals.
Infectious substances meeting these criteria which cause disease in humans or both in humans and animals shall be assigned to UN 2814. Infectious substances which cause disease only in animals shall be assigned to UN 2900.
Assignment to UN 2814 or UN 2900 shall be based on the known medical history and symptoms of the source human or animal, endemic local conditions, or professional judgment concerning individual circumstances of the source human or animal.
NOTES:
Packing instruction P620 (UN) or PI 602 (IATA) apply to these substances.
NOTE: Figure 3 shows examples of triple packaging systems for Category A and Category B infectious substances.
Table 13.1 is not exhaustive. Infectious substances, including new or emerging pathogens, which do not appear in the Table but which meet the same criteria shall be assigned to Category A. In addition, if there is any doubt as to whether or not a substance meets the criteria, it shall be included in Category A.
132| UN 2814 Infectious substance affecting humans | |
|---|---|
| Bacillus anthracis (cultures only) | Human immunodeficiency virus (cultures only) |
| Brucella abortus (cultures only) | Japanese Encephalitis virus (cultures only) |
| Brucella melitensis (cultures only) | Junin virus |
| Brucella suis (cultures only) | Kyasanur Forest disease virus |
| Burkholderia mallei, Pseudomonas mallei, Glanders (cultures only) | Lassa virus Machupo virus |
| Burkholderia pseudomallei, Pseudomonas pseudomallei (cultures only) | Marburg virus |
| Chlamydia psittaci, avian strains (cultures only) | Monkeypox virus |
| Clostridium botulinum (cultures only) | Mycobacterium tuberculosis (cultures only) |
| Coccidioides immitis (cultures only) | Nipah virus |
| Coxiella burnetti (cultures only) | Omsk haemorrhagic fever virus |
| Crimean-Congo haemorrhagic fever virus | Poliovirus (cultures only) |
| Dengue virus (cultures only) | Rabies virus (cultures only) |
| Eastern equine encephalitis virus (cultures only) | Rickettsia prowazekii (cultures only) |
| Ebola virus | Rickettsia rickettsii (cultures only) |
| Escherichia coli, verotoxigenic (cultures only) | Rift Valley fever virus (cultures only) |
| Flexal virus | Russian spring-summer encephalitis virus (cultures only) |
| Francisella tularensis (cultures only) | Sabia virus |
| Guanarito virus | Shigella dysenteriae type 1 (cultures only) |
| Hantaan virus | Tick-borne encephalitis virus (cultures only) |
| Hantavirus causing haemorrhagic fever with renal syndrome | Variola virus Venezuelan equire encephalitis virus (cultures only) |
| Hendra virus | West Nile virus (cultures only) |
| Hepatitis B virus (cultures only) | Yellow fever virus (cultures only) |
| Herpes B virus (cultures only) | Yersinia pestis (cultures only) |
| Highly pathogenic avian influenza virus (cultures only) | |
| UN 2900 Infectious substance affecting animals | |
| African swine fever virus (cultures only) | Peste des petits ruminants virus (cultures only) |
| Avian paramyxovirus Type I, Velogenic Newcastle disease virus (cultures only) | Rinderpest virus (cultures only) |
| Classical swine fever virus (cultures only) | Sheep-pox virus (cultures only) |
| Foot and mouth disease (cultures only) | Goat-pox virus (cultures only) |
| Lumpy skin disease virus (cultures only) | Swine vesicular disease virus (cultures only) |
| Mycoplasma mycoides, Contagious bovine pleuropneumonia (cultures only) | Vesicular stomatitis virus (cultures only) |
An infectious substance which does not meet the criteria for inclusion in Category A. Infectious substances in Category B shall be assigned to UN 3373.
NOTE: The proper shipping name of UN 3373 is BIOLOGICAL SUBSTANCE, CATEGORY B. The shipping name DIAGNOSTIC SPECIMENS, CLINICAL SPECIMENS has been phased out.
Packing instruction P650 (UN) or PI 650 (IATA) apply to these substances.
NOTE: Figure 3 shows examples of triple packaging systems for Category A and Category B infectious substances.
Category C applies to surface transport in Australia only. Patient specimens including excreta, secreta, blood and its components, tissues and tissue fluids and biological materials with a low probability of causing disease in humans, animals and plants that could cause community concerns if the specimen was to leak from its packaging fall into Category C in AS 4834 and, if transported by land, shall be packaged, marked, documented and transported according to the requirements in AS 4834. If transported by air, IATA regulations for exempt patient specimens shall be followed.
The current edition of the IATA Dangerous Goods Regulations shall be consulted.
Biological products are divided into the following groups:
NOTE: Some licensed biological products may present a biohazard only in certain parts of the world. In that case, competent authorities may require these biological products to be in compliance with local requirements for infectious substances or may impose other restrictions.
Genetically modified microorganisms shall be transported according to the guidelines or standards published by the OGTR or MAF, as appropriate.
Medical or clinical wastes containing Category A infectious substances shall be assigned to UN 2814 or UN 2900 as appropriate. Medical or clinical wastes containing infectious substances in Category B, shall be assigned to UN 3291.
Medical or clinical wastes which are reasonably believed to have a low probability of containing infectious substances shall be assigned to UN 3291.
NOTE: The proper shipping name for UN 3291 is CLINICAL WASTE, UNSPECIFIED, N.O.S. or (BIO) MEDICAL WASTE, N.O.S. or REGULATED MEDICAL WASTE, N.O.S.
Packing instruction P622 applies to medical or clinical wastes.
134Decontaminated medical or clinical wastes that previously contained infectious substances arc not subject to the UN Model Regulations unless they meet the criteria for inclusion in another class.
NOTE: AS/NZS 3816 should also be consulted.
A live animal that is known or suspected to contain an infectious substance shall not be transported by air unless the infectious substance contained cannot be transported by any other means. Infected animals may only be transported under terms and conditions approved by the competent authority.
Animal carcasses affected by pathogens of Category A or which would be assigned to Category A in cultures only, shall be assigned UN 2814 or UN 2900 as appropriate. Other animal carcasses affected by pathogens included in Category B shall be transported in accordance with provisions determined by the competent authority.
When infectious material is being transported, a Shipper’s Declaration for Dangerous Goods shall be completed indicating origin, contents and date of dispatch, and shall be attached to the external surface of the package. Documentation enclosed in a package shall be placed between the primary and secondary packages, and inside outer packaging, in a separate impervious bag to protect it from contamination by contents of the package. Recipients shall be informed of all known hazards associated with the material in advance of delivery.
135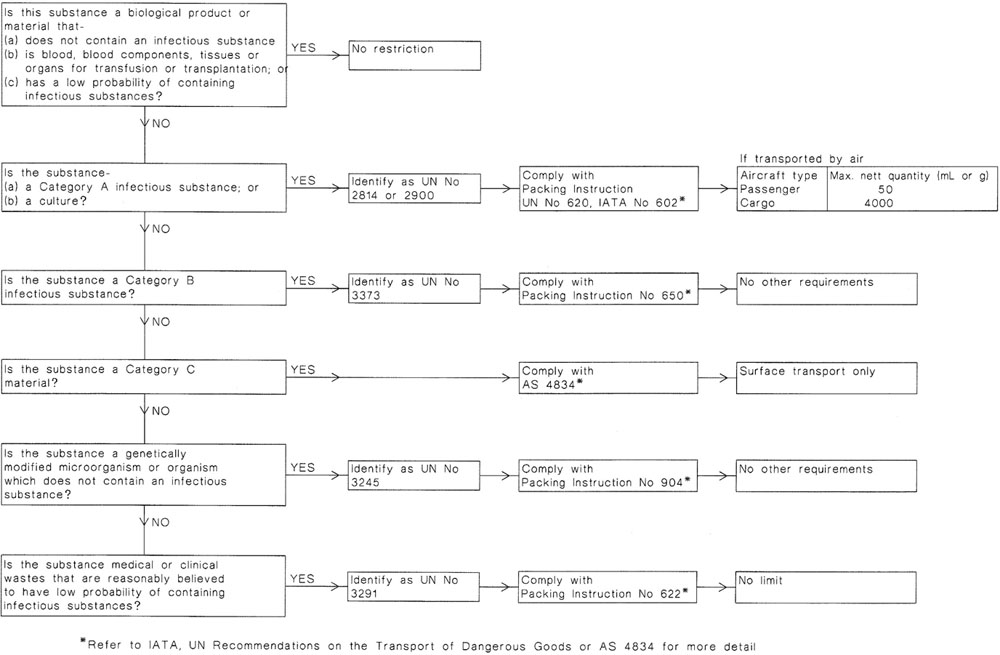
FIGURE 2 SUMMARY OF BIOLOGICAL MATERIAL PACKAGING INSTRUCTION CLASSIFICATION
136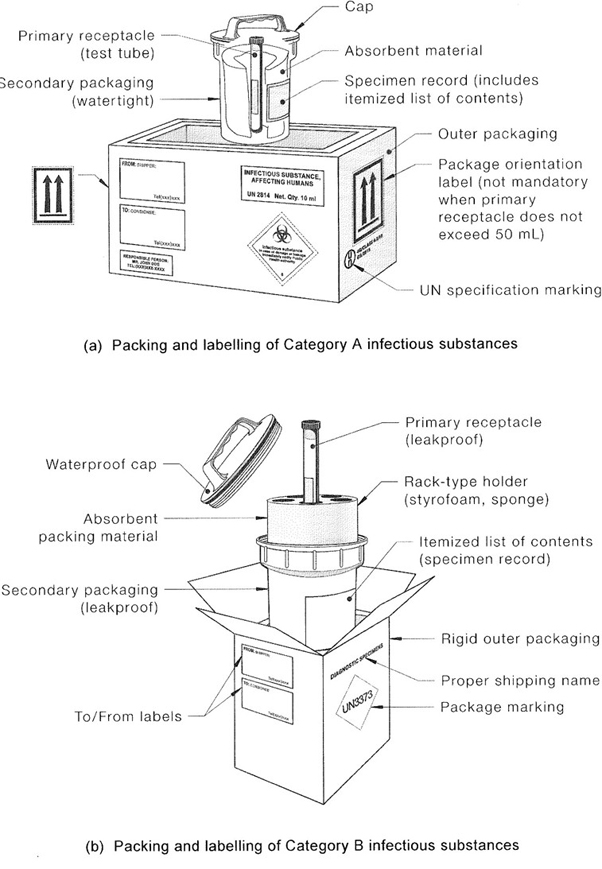
FIGURE 3 EXAMPLES OF TRIPLE PACKAGING SYSTEMS
137(Normative)
This Appendix provides lists of documents and publications referred to in this Standard, as well as a list of related publications.
The following documents are referred to in this Standard:
| AS | |
| 1319 | Safety signs for the occupational environment |
| 1324 | Air filters for use in general ventilation and airconditioning |
| 1324.1 | Part 1: Application, performance and construction |
| 1324.2 | Part 2: Methods of test |
| 1386 | Cleanrooms and clean workstations |
| 1386.5 | Part 5: Clean workstations |
| 1410 | Sterilizers—Steam—Pre-vacuum |
| 1668 | The use of ventilation and airconditioning in buildings |
| 1668.2 | Part 2: Ventilation design for indoor air contaminant control |
| 1807 | Cleanrooms, workstations, safety cabinets and pharmaceutical isolators—Methods of test |
| 1807.6 | Method 6: Determination of integrity of terminally mounted HEPA filter installations |
| 1807.7 | Method 7: Determination of integrity of HEPA filter installations not terminally mounted |
| 1807.10 | Method 10: Determination of air pressure of cleanrooms and pharmaceutical isolators |
| 2192 | Sterilizers—Steam—Downvard—displacement |
| 2243 | Safety in laboratories |
| 2243.4 | Part 4: Ionizing radiations |
| 2252 | Biological safety cabinets |
| 2252.1 | Part 1: Biological safety cabinets (Class I) for personnel and environment protection |
| 2252 | Controlled environments |
| 2252.2 | Part 2: Biological safety cabinets (Class II)—Design |
| 2567 | Laminar flow cytotoxic drug safety cabinets |
| 2639 | Laminar flow cytotoxic drug safety cabinets—Installation and use |
| 4031 | Non-reusable containers for the collection of sharp medical items used in health care areas 138 |
| AS | |
| 4260 | High efficiency particulate air (HEPA) filters—Classification, construction and performance |
| 4381 | Single-use face masks for use in health care |
| 4834 | Packaging for surface transport of biological material that may cause disease in humans, animals and plants |
| AS/NZS | |
| 1170 | Structural design actions |
| 1170.2 | Part 2: Wind actions |
| 1336 | Recommended practices for occupational eye protection |
| 1337 | Personal eye protection (series) |
| 1338 | Filters for eye protectors (series) |
| 1715 | Selection, use and maintenance of respiratory protective devices |
| 2161 | Occupational protective gloves (series) |
| 2210 | Occupational protective footwear (series) |
| 2243 | Safety in laboratories |
| 2243.1 | Part 1: Planning and operational aspects |
| 2243.2 | Part 2: Chemical aspects |
| 2243.8 | Part 8: Fume cupboards |
| 2243.9 | Part 9: Recirculating fume cabinets |
| 2243.10 | Part 10: Storage of chemicals |
| 2647 | Biological safety cabinets—Installation and use |
| 2982 | Laboratory design and construction |
| 2982.1 | Part 1: General requirements |
| 3500 | Plumbing and drainage (series) |
| 3816 | Management of clinical and related wastes |
| AS/NZS ISO | |
| 31000 | Risk management—Principles and guidelines |
| ISO | |
| 3864 | Graphical symbols—Safety colours and safety signs (series) |
| NZS | |
| 4304 | Management of healthcare waste |
| 5433 | Transport of dangerous goods on land |
| ACTDG (Advisory Committee on the Transport of Dangerous Goods, Australia) ADG Code, Australian Code for the Transport of Dangerous Goods by Road and Rail |
|
| CWA (CEN Workshop Agreement) | |
| 15793 | Laboratory Biorisk Management Standard (available from www.cen.eu/cenorm/businessdoniains/technicaleommitteesworkshops/workshops/ws31.asp) |
| CIVIL AVIATION SAFETY AUTHORITY (Australia) Civil Aviation Safety Regulations 1998 |
|
| COMMONWEALTH OF AUSTRALIA National Health Security Act 2007 |
|
DEPARTMENT OF HEALTH AND AGEING, AUSTRALIA
Infection control guidelines for the prevention of transmission of infectious diseases in the health care setting
IATA (International Air Transport Association)
Dangerous Goods Regulations
IMO (International Maritime Organization)
International Maritime Dangerous Goods Code
MAF BNZ—ERMA, New Zealand
Facilities for Microorganisms and Cell Cultures:2007a
MINISTRY OF HEALTH NEW ZEALAND
Guidelines for Tuberculosis Control in New Zealand 2003
Immunisation Handbook 2006
NHMRC (National Health and Medical Research Council, Australia)
Australian code of practice for the care and use of animals for scientific purposes
The Australian Immunisation Handbook
Guidelines to promote the wellbeing of animals used for scientific purposes: The assessment and alleviation of pain and distress in research animals
| NOHSC (National Occupational Health and Safety Commission) | |
| 2010 | National Code of Practice for the control of work related exposure to hepatitis and HIV (blood-borne) viruses |
| NRL (National Radiation Laboratory, NZ) | |
| C1 | Code of safe practice for the use of unsealed radioactive materials |
NTAC (National Tuberculosis Advisory Committee, Australia)
Guidelines for Australian Mycobacteriology Laboratories
New Zealand Animal Welfare Act 1999
New Zealand Hazardous Substances and New Organisms Act 1996
OGTR (Office of the Gene Technology Regulator, Australia)
Guidelines for the certification of physical containment facilities
Guidelines for the transport of GMOs
UNITED NATIONS
Recommendations on the Transport of Dangerous Goods. Model Regulations
The following publications are referred to in this Standard:
| 1.1 | CENTERS FOR DISEASE CONTROL AND PREVENTION, Guidelines for the investigation of contacts of persons with infectious tuberculosis: recommendations from the National Tuberculosis Controllers Association and CDC, and Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection. Morbidity and Mortality Weekly Review 54 (Recomrnendations and Reports 15) 2005; p. 1-62. |
| 1.2 | COLLINS, C.H. and KENNEDY, D.A. (eds.) Laboratory-acquired infections: history, incidence, causes and prevention. 4th ed. Oxford: Butterworth Heinemann, 1999. |
| 1.3 | US DEPARTMENT OF HEALTH AND HUMAN SERVICES. Biosafety in microbiological and biomedical laboratories. 5th ed. Washington D.C.: US Government Printing Office, 2007. 140 |
| 1.4 | UK ADVISORY COMMITTEE ON DANGEROUS PATHOGENS. The management, design and operation of microbiological containment laboratories. London: HSE Books, 2001. |
| 1.5 | PUBLIC HEALTH AGENCY CANADA. Laboratory biosafety guidelines. 3rd ed. Ottawa: Population and Public Health Branch, Centre for Emergency Preparedness and Response, 2004. |
| 1.6 | WORLD HEALTH ORGANIZATION. WHO/CDS/CSR/LYO/2004.11 Laboratory biosafety manual. 3rd ed. Geneva: World Health Organization, 2004. (Available from WHO web site at www.who.int/csr/resources/publicatiuons). |
| 1.7 | DR VRIES, B. and COSS ART, Y.E. Needlestick injury in medical students. Medical Journal of Australia. 1994; vol. 160: p. 398-400. |
| 1.8 | NATIONAL INSTITUTE FOR OCCUPATIONAL SAFETY AND HEALTH. Preventing Asthma in Animal Handlers. Publication No. 97-116, January 1998. |
| 1.9 | BOYCE J.M. and PITTET M.D., Guideline for Hand Hygiene in Health Care Settings Morbidity and Mortality Weekly Review 51(Recommendations and Reports 16) 25 October 2002; p. I-44. |
| 1.10 | L ENHART. S.W., SEITZ, T., TROUT, D. and BOLLINGER, N. Issues affecting respirator selection for workers exposed to infectious aerosols: Emphasis on healthcare settings. Applied Biosafety 2004; vol. 9(1): p. 20-36. |
| 1.11 | McCULLOUGH, N. Personal respiratory protection. In FLEMING, D.O. and HUNT, D.L. (eds.). Biological safety: Principles and practices. 4th ed. Washington D.C.: American Society for Microbiology, 2006. |
| 1.12 | NATIONAL OCCUPATIONAL HEALTH AND SAFETY COMMISSION. Adopted National exposure standards for atmospheric contaminants in the occupational environment. 3rd ed. Canberra: Australian Government Publishing Service. 1995. |
| 1.13 | LAVOIE, J., CLOUTIER, Y., LARA, J. and MARCHAND, G. Technical Guide RG-501: Guide on respiratory protection against bioaerosols: Recommendations on its selection and use. Montreal: Occupational Health and Safety Research Institute Robert-Sauvé, 2007. |
| 1.14 | GARDNER, J.F. and PEEL, M.M. Sterilization, disinfection and infection control. 3rd ed. Melbourne: Churchill Livingstone, 1998. |
| 1.15 | GARLAND, A.J.M. A review of BSE and its inactivation. European Journal of Parenteral Sciences. 1999; vol. 4: p. 86-93. |
| 1.16 | WORLD HEALTH ORGANIZATION. WHO Infection Guidelines for Transmissible Spongiform Encephalopathies. Report of a Consultation. March 1999. Geneva: World Health Organization, 2000. |
| 1.17 | BARBEITO, M.S. and SHAPIRO, M. Microbiological safety evaluation of a solid and liquid pathological incinerator. Journal of Medical Primatology. 1977; vol. 6: p. 264-73. |
| 1.18 | AUSTRALIA POST. Dangerous and prohibited goods packaging guide. Post Guide. Section 2.5.15, Melbourne: Australia Post, 1996. |
| 1.19 | NEW ZEALAND POST. Postal users guide. Wellington: New Zealand Post, 1994. |
| 1.20 | BLOOM FIELD, S.F., SMITH-BURCHNELL, C.A. and DALGLEISH, A.G. Evaluation of hypochlorite-releasing disinfectants against the human immunodeficiency virus (HIV). Journal of Hospital Infection. 1990; vol. 15: p. 273-8. 141 |
| 1.21 | ABRAHAM, G., LE BLANC SMITH, P.M. and NGUYEN, S. The effectiveness of gaseous formaldehyde decontamination assessed by biological monitoring. Journal of the American Biological Safety Association 1997; vol. 2(1): p. 30-8. |
| 1.22 | DRUCE, J.D., JARDINE, D., LOCARNINI, S.A., and BIRCH, C.J. Susceptibility of HIV to inactivation by disinfectants and ultraviolet light. Journal of Hospital Infection. 1995; vol. 30: p. 167-80. |
| 1.23 | BROADLEY, S.J., FURR, J.R., JENKINS, P.A., and RUSSELL, A.D. Antimycobacterial activity of ‘Virkon’. Journal of Hospital Infection. 1993; vol 23: p. 189-97. |
| 1.24 | MONTEFIORI, D.C., ROBINSON, W.E.Jr., MODLISZEWSKI, A. and MITCHELL, W.M. Effective inactivation of human immunodeficiency virus with chlorhexidine antiseptics containing detergents and alcohol. Journal of Hospital Infection. 1990; vol. 15: p. 279-82. |
| 1.25 | RUTALA, W. and WEBER, D. New Disinfection and Sterilisation Methods. Emerging Infectious Diseases. 2001; vol. 7 (2): p. 348-353. |
| 1.26 | RIDEOUT, K., TESCHKE, K., DIMICH-WARD, H. and KENNEDY, S. Considering the risk to healthcare workers from glutaraldehyde alternatives in high-level disinfection. Journal of Hospital Infection. 2005; vol. 59(1): p. 4-11. |
| 1.27 | MCGURK, G.B. A study of of air-tightness in Australian high-level bio-containment facilities. Journal of the American Biological Safety Association 2009; vol. 14 (2):p. 72-80. |
| 1.28 | CZARNESKI, M. A. Microbial Decontamination of a New 65-Room Pharmaceutical Research Facility. Journal of the American Biological Safety Association 2009; vol. 14(2): pp. 81-88. |
| 1.29 | CETA Application Guide for the use of Surface Decontaminants in Biosafety Cabinets, CAG-004-2007, www.cetainternational.org/reference/CAG0042007i.pdf |
| 1.30 | BABB, J.R., BRADLEY, C.R. and AYLIFFE G.A.J. Sporicidal activity of glutaraldehydes and hypochlorites and other factors influencing their selection for the treatment of medical equipment, J Hosp Infect. 1980; vol 1 :p 63-75. |
| 1.31 | FICHET, G., ANTLOGA, K., COMOY, E., DESLYS, J.P. and MCDONNELL G. Prion inactivation using a new gaseous hydrogen peroxide sterilisation process. J Hosp Infect. 2007 Nov; vol. 67(3):p 278-86. |
| 1.32 | BEST, M., SATTAR. S.A., SPRINGTHORPE, V. S. and KENNEDY, M.E. Efficacies of selected disinfectants against Mycobacterium tuberculosis, J Clin Microbiol. (1990) October; vol. 28 (10):p 2234-2239. |
| 1.33 | FICHET, G., COMOY, E., DUVAL, C., ANTLOGA, K., DEHEN, C., CHARBONNIER, A., MCDONNELL, G., BROWN, P., LASMEZAS, C.I., and DESLYS, J.P., Novel methods for disinfection of prion-contaminated medical devices. Lancet . 2004; Aug 7-13:364(9433):p 521-6. |
Attention is drawn to the following related documents:
| 2.1 | ABRAHAM. G. and DELLA-PORTA. A.J. Microbiological containment and biosafety in medical and veterinary laboratories. Recent advances in microbiology. 1993; vol. 1: p. 228-58. |
| 2.2 | AYLIFFE, G.A.J., COATES, D. and HOFFMAN, P.N. Chemical disinfection in hospitals. 2nd ed. London: Public Health Laboratory Service, 1993. 142 |
| 2.3 | BLOCK, S.S. (ed.). Disinfection, sterilization, and preservation. 5th ed. Philadelphia: Lippincott Williams and Wilkins, 2001. |
| 2.4 | FLEMING, D.O. and HUNT, D.L. (eds.). Biological safety: Principles and practices. 4th ed. Washington D.C.: American Society for Microbiology, 2006. |
| 2.5 | FURR, A.K. (ed.). CRC Handbook of laboratory safety. 3rd ed. Boca Raton, Florida: CRC Press, 1990. |
| 2.6 | LAUER, J.L., BATTLES, D.R. and VESLEY, D. Decontaminating infectious laboratory waste by autoclaving. Applied and Environmental Microbiology. 1982; vol. 44: no 3, p. 690-4. |
| 2.7 | NATIONAL HEALTH AND MEDICAL RESEARCH COUNCIL. National guidelines for the management of clinical and related wastes. Canberra: Australian Government Publishing Service, 1989. |
| 2.8 | RUSSELL, A.D., HUGO, W.B. and AYLIFFE, G.A. (eds.). Principles and practices of disinfection, preservation and sterilization. 3rd ed. Oxford: Blackwell Scientific Publications, 1999. |
| 2.9 | SAX, N.I. Dangerous properties of industrial materials. 10th ed. New York: Van Nostrand Reinhold, 2000. |
| 2.10 | POOLE, T. (ed.). The UFAW Handbook on the care and management of laboratory animals. 6th ed. Great Britain, Bath Press, 1987. |
| 2.11 | RICHMOND, J.Y. (ed.). Anthology of Biosafety (series). American Biological Safety Association, 1999-. |
| 2.12 | PHILBEY, A.W., KIRKLAND, P.D., ROSS, A.D., DAVIS, R.J., GLEESON, A.B., LOVE, R.J., DANIELS, P.W., GOULD, A.R. and HYATT, A.D. An apparently new virus (Family Paramyxoviridae) infectious for pigs, humans and fruit bats. Emerging Infectious Diseases. 1998; vol. 4, no. 2: p. 269-71. |
| 2.13 | UK ADVISORY COMMITTEE ON DANGEROUS PATHOGENS. Guidance on the use, testing and maintenance of laboratory and animal flexible film isolators. London: HSE Books, 1985.
NOTE: Information on risk groups from the UK health and Safety Executive is continually being updated and may be found at their website, e.g. www.hse.gov.uk/pubns/misc208.pdf |
| 2.14 | COMMONWEALTH OF AUSTRALIA. Gene Technology Act 2000. |
| 2.15 | COMMONWELATH OF AUSTRALIA. Gene Technology Regulations 2001. |
| 2.16 | STANDARDS AUSTRALIA. AS/NZS 4187. Cleaning, disinfecting and sterilizing reusable medical and surgical instruments and equipment, and maintenance of associated environments in health care facilities. Sydney: Standards Australia 2003. |
| 2.17 | BUCHEN-OSMOND, C., CRABTREE, K., GIBBS, A. and MCLEAN, G. (eds.). Viruses of plants in Australia. Canberra: The Australian National University Printing Service, 1988. |
| 2.18 | KAHN, R.P. and MATHUR, S.B. Containment facilities and safeguards for exotic plant pathogens and pests. American Phytopathological Society, 1999. |
| 2.19 | CSL Limited. Q fever: your questions answered. Sydney: MediMedia Communications, 1999. |
| 2.20 | WORLD HEALTH ORGANIZATION. Guidance on regulations for the transport of infectious substances. WHO/CDS/CSRL/LYO/2005.22. Lyons: World Health Organization, 2005. |
(Informative)
__________________________________________________________
_____________________________________________________
What was the employee doing and how did the incident exposure occur? (Describe the work being performed, list sequence of events).
_____________________________________________________
_____________________________________________________
_____________________________________________________
_____________________________________________________
Name of microorganism(s)__________________________________
Risk group: _____________________________________________
Nature of genetic modification: _______________________________
(Names)
_____________________________________________________
_____________________________________________________
_____________________________________________________
(Include names of personnel involved, personal protective equipment and disinfectant used).
_____________________________________________________
_____________________________________________________
(Names) 1.________________________ 2.___________________
State what witness saw happen.
_____________________________________________________
_____________________________________________________
_____________________________________________________
Name: Signature: Date:
_____________________________________________________
_____________________________________________________
Actioning officer: Completion date: Signature:
(Normative)
This Appendix sets out additional requirements for working with poliovirus.
The World Health Organization (WHO) has issued guidance documents* related to work with wild poliovirus in the near and long-term future.
People wishing to work with wild poliovirus during containment Phase 1, Laboratory Survey and Inventory, during which wild polioviruses are decreasing world-wide, shall do so under PC2/polio containment. PC2/polio containment follows traditional PC2 requirements for facilities, practices and procedures, with the additional requirements that—
Unless there are strong scientific reasons for working with virulent polioviruses, laboratories should use the attenuated Sabin oral poliovirus vaccine (OPV) strains. Authenticated OPV strains can be supplied on application to the National Polio Reference Laboratory, Victorian Infectious Diseases Reference Laboratory, Australia or Environmental Science and Research, New Zealand. Further information on the eradication and containment of wild polioviruses is available at www.polioeradication.org and www.polioeradication.org/content/publications/WHO-VB-03-729.pdf.
All laboratories retaining wild poliovirus infectious materials or potential wild poliovirus infectious materials should be listed on the inventory held by the national government. The
*WORLD HEALTH ORGANIZATION. WHO global action plan for laboratory containment of wild polioviruses. 2nd ed. WHO/V and B/03/11 Geneva: World Health Organization. 2003.
145purpose of the inventory is to meet the country requirements defined by WHO for the certification of regions as polio-free, and to maintain a current list of laboratories to be notified about initiating the appropriate containment procedures one year after the global detection of the last wild poliovirus. The inventory is updated regularly and submitted for review and endorsement to the National and Regional Commissions for Certification of Polio Eradication. The Australian inventory also includes laboratories using OPV strains. The contacts are for Australia: www.health.gov.au and for New Zealand: www.moh.gov.nz.
Phase II, Global Certification, begins one year after no case has been detected globally. At this time, all laboratories wishing to retain wild poliovirus infectious or potentially infectious materials shall begin implementing biosafety measures appropriate for the laboratory procedures being performed (PC2/polio or PC3/polio containment procedures). All activities involving wild polio infectious materials, including storage, shall be carried out under PC3/polio containment. All activities that involve inoculating poliovirus-permissive cells or animals with potential wild polio infectious materials shall also be performed under PC3/polio containment. Other activities involving potential wild polio infectious materials may be conducted safely in a certified class I or II biological safety cabinet in a PC2/polio laboratory. Centrifuging such materials may be done in an open laboratory if sealed rotors or safety cups are used and these are only loaded and opened in a biological safety cabinet. A PC3/polio laboratory shall incorporate all the microbiological practices and procedures described for the PC2/polio laboratory. Major facility requirements are described in Clause 5.4.2. Alternatively, laboratories may contact a laboratory capable of meeting the required biosafety standards to arrange for transfer and storage of selected materials.
After two years passes with no reported cases globally, containment should be completed and documentation of implementation submitted. Global polio eradication will be certified after three years with no cases reported.
Phase III, Post Global Certification refers to a time in the future when post-eradication data and experiences suggest to some countries the need to consider the option of discontinuing polio immunization. When oral poliovirus vaccine immunization stops, with or without universal replacement with inactivated polio vaccine (IPV), the biosafety requirements for both wild and oral polio vaccine (OPV) viruses will become more stringent than those outlined in the second edition of the Global Action Plan, consistent with the consequences of inadvertent transmission of poliovirus from the laboratory to an increasingly susceptible community.
146(Normative)
The biological hazard symbol shown in Figure D1 is specified in AS 1319, and is recognized worldwide, e.g. by the World Health Organization and the United Nations Committee on the Transport of Dangerous Goods. These signs are readily available from commercial sources of laboratory or medical supplies.
The colour scheme for signs incorporating the biological hazard symbol shall be a black symbol on a yellow background, as specified in AS 1319 and ISO 3864 for all warning signs.
FIGURE D1 BIOLOGICAL HAZARD SYMBOL
147The sign for general microbiological laboratories shall be in the format shown in Figure D2, i.e. it shall show the biological hazard symbol shown in Figure Dl and the laboratory containment level. The colours used in the sign shall be black for the symbol and writing on a yellow background as specified for safety signs in AS 1319 and ISO 3864.

FIGURE D2 LAYOUT FOR GENERAL MICROBIOLOGICAL LABORATORY WARNING SIGN (EXAMPLE FOR PC2 LABORATORY)
148(Normative)
This Appendix provides requirements for the prevention of contamination of water and gas supplies, prevention of cross-contamination between different level facilities and general gas supply requirements.
In addition to high hazard rating boundary containment protection in accordance with AS/NZS 3500 and local authority requirements, individual backflow prevention devices to suit a high hazard rating situation shall be installed in the following water supply lines as illustrated in Figure El:
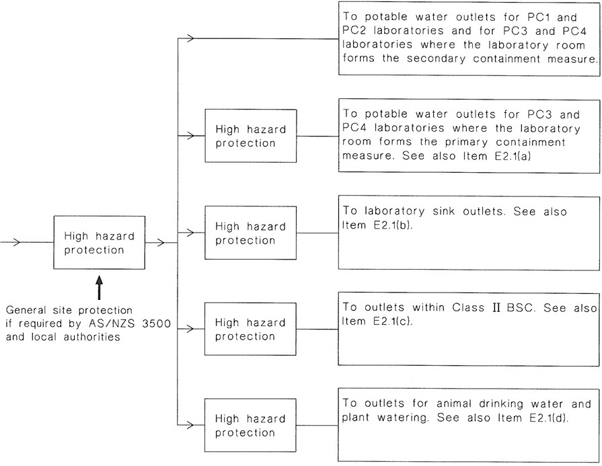
FIGURE E1 BACKFLOW PREVENTION FOR LABORATORY WATER SUPPLIES
For PC1, PC2, PC3 and PC4 facilities where the room forms the secondary containment measure, looped service outlets other than those outlets connected to primary containment equipment and those in BSC Class II should be provided with the minimum piping inside the facility.
For BSC Class II and within PC3 and PC4 facilities where the room forms the primary containment measure (e.g. large animal facilities, plant facilities), looped service outlets should be avoided. The preferred method of supplying water is by carrying it in using containers that can be decontaminated using pressure steam sterilization. If outlets are provided they shall have backflow prevention in the form of one of the following options, preferably that in Item (a):
All looped service outlets shall be accompanied by a sign containing the wording ‘Looped service outlet: Maintain an air gap at all times’.
For sealed loop services, such as cooling water loops and sealed steam/condensate circuits, no backflow prevention is required. Systems shall be tested at least annually to confirm that the seal is maintained. Isolation shall be provided within the facility for service and maintenance. For facilities that require gaseous decontamination capability, systems shall be capable of withstanding gaseous decontamination in both assembled and dismantled states.
150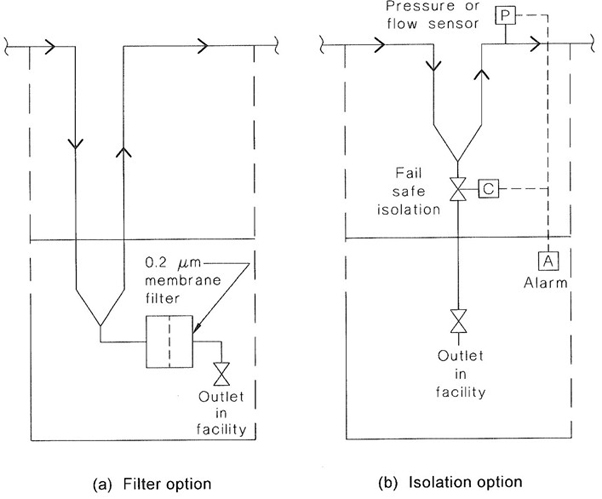
FIGURE E2 BACKFLOW PREVENTION FOR LOOPED SERVICE OUTLETS
The following general requirements apply to gas services in microbiological containment facilities:
Reverse flow protection shall be provided between the facility’s piped gas service and outlets in the following gas services as illustrated in Figure E3:
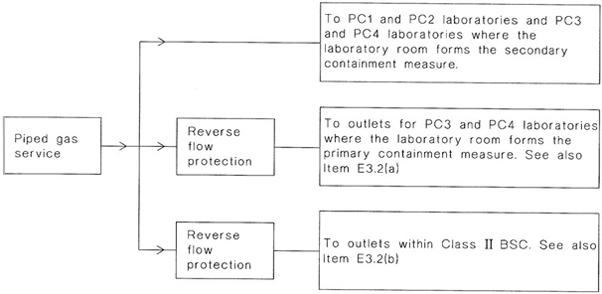
FIGURE E3 BACKFLOW PREVENTION FOR GAS SERVICES
152(Informative)
Prior to any form of disinfection or decontamination, equipment and surfaces should where possible be cleaned and free from organic material and grease. Pressure steam sterilization (autoclaving) is the most reliable means of decontamination. However, this method is not applicable in all situations. Chemical disinfection is often the only practical method of decontamination for large spaces or surface areas and for heat-labile materials or equipment. Mechanical brushing and rubbing facilitate the action of chemical disinfectants and other forms of decontamination. Where time permits, heat-labile materials and equipment may be sterilized by gaseous chemicals such as ethylene oxide or by ionizing radiation. For a general guide to the use of disinfectants see Ref 1.14.
Microorganisms vary in their susceptibility to chemical disinfectants. Lipid-containing viruses and the vegetative forms of most bacteria are relatively susceptible. Fungi, acid-fast bacteria (Mycobacterium spp.) and non-lipid-containing viruses are less susceptible while bacterial spores are resistant to the action of many chemical disinfectants. The agents of scrapie. Creutzfeldt-Jakob disease and other prions are extremely resistant to chemical disinfection (see Clauses 3.7 and 12.2.1).
Many chemical disinfectants are available under a variety of trade names. Examples of chemical disinfectants with a broad spectrum of activity against a range of microorganisms, including some sporicidal activity, are as follows:
Chemical disinfectants with a more limited antimicrobial spectrum include the following:
Variables that may affect the action of chemical disinfectants include the following:
The choice of a chemical disinfectant often represents a compromise between the requirement for a broad antimicrobial spectrum, the limitations imposed by the situation or type of materials being disinfected, and any disadvantages of particular disinfectants. A chemical disinfectant which is suitable for a particular purpose or situation depends not only on the types of microorganisms likely to be present but also on the control or provision of the conditions that can promote its effectiveness in that situation. Other properties of the disinfectant also need to be considered, such as possible corrosive, bleaching or staining effects and its flammability. In addition, the effect it can have on personnel as a toxic irritant, any sensitizing action and its carcinogenic potential need to be taken into account.
Material safety data sheets (MSDS) should be obtained from the supplier or distributor for any chemical disinfectant used in the workplace. A request for the relevant MSDS should automatically accompany the initial order for materials. MSDS provide information on the identity, physical characteristics, potential health hazards and precautions to be taken for safe storage, use and disposal of chemicals. The laboratory supervisor should ensure that all persons have access to MSDS for the substances that are used in the workplace and that these are read and understood by those concerned. MSDS, as obtained from suppliers, should not be altered although additional information may be appended and clearly marked as such.
Tables F1 and F2 should be consulted for assistance when selecting disinfectants. Table F1 provides recommended applications for chemical disinfectants in microbiological facilities.
Table F2 provides a guide to the effectiveness of different classes of disinfectant against a range of microorganisms (see Reference 1.29). The disinfectants in Table F2 have been categorised according to the range of microorganisms that they are able to inactivate under optimum conditions:
Tables F1 and F2 are informative only. Although the disinfectants are considered to be effective against all the microorganisms within a particular group where stated, the contact time required to inactivate different microorganisms within a group can vary. This is particularly so where inactivation of bacterial endospores is required. The effectiveness of any disinfection processes should be validated against the microorganism of choice and under the conditions in which the disinfection should occur.
In the form of sodium hypochlorite or other chlorine-releasing compounds, chlorine is active against vegetative forms of bacteria and viruses and is the preferred chemical disinfectant for HIV and hepatitis viruses. It is less effective against spores. Chlorine combines rapidly with proteins, so, in the presence of organic materials, the concentration of chlorine needs to be increased to overcome this organic demand. For example, an equal
154volume of 5000–10 000 p.p.m. (0.5–1%) available chlorine is required for the inactivation of HIV and hepatities viruses in blood or serum (see Reference 1.20).
Commercially available chlorine solutions vary in the concentration of available chlorine they contain. For example, some solutions contain 4% (e.g. household beach) while others contain 12.5% available chlorine. Care should be taken when diluting these solutions to ensure the correct final working concentration is achieved
NOTE: Information on diluting chlorine-containing solutions for disinfection purposes is provided in Attachment 4 to the Victorian Department of Human Services publication ‘Guidelines for the investigation of gastrointestinal illness’ available at web address: www.health.vic.gov.au/ideas/diseases/gas_ill_index.htm
As the effective strength of chlorine solutions decreases on storage, working solutions should be freshly prepared each day. Stabilized solutions of sodium hypochlorite with added sodium chloride are preferred as these solutions maintain a greater effective chlorine concentration. For effective biocidal action, a pH range of 6–8 is optimum. High concentrations of hypochlorite solutions are corrosive to stainless steel and other metal surfaces and tend to bleach and damage fabrics.
A cheap and useful decontaminant with good wetting properties can be prepared by adding a non-ionic detergent to a solution containing about 500 p.p.m. (0.05%) of available chlorine to give a detergent concentration of 0.7% v/v. This solution is suitable for disinfecting contaminated pipettes.
Iodine, in aqueous or alcoholic solution, has a wide spectrum of antimicrobial activity including some sporicidal action. It has the disadvantage of staining skin and may cause irritation and sensitization.
Iodophors are organic compounds of surface active agents and iodine which rely on the slow release of iodine for activity. Free iodine reacts more slowly with organic matter than does chlorine but inactivation may be significant in dilute iodine solutions. The optimum pH for activity is in the neutral to acid range. Decomposition occurs at temperatures above 40°C with the release of iodine vapour which is toxic on absorption. Povidone-iodine is used as a skin disinfectant.
A solution of about 37% w/v formaldehyde gas in water is known as formalin. A solution of 5% w/v formaldehyde, i.e. about 13% v/v formalin, is a good decontaminant but it has a strong, irritating odour. Solutions of 8% v/v formalin in 80% v/v alcohol are considered to be very good for disinfection purposes because of their effectiveness against vegetative bacteria, spores and viruses. Formaldehyde is also available in polymerized form, known as paraformaldehyde, which, on heating, decomposes to formaldehyde gas.
Precautions are necessary for handling formaldehyde and when entering rooms which have been decontaminated by gaseous formaldehyde as it is a highly toxic gas and is classified as a known human carcinogen. The Australian National Exposure Standard, expressed as a TWA, for formaldehyde is specified as I p.p.m. or 1.2 mg/m3 (see Reference 1.12) and is currently under review. Formaldehyde and paraformaldehyde should only be opened or weighed in a fume cupboard. Under certain conditions, formaldehyde can react with free chlorine or chloride ions to form an unstable compound, bis (chloromethyl) ether, which is a potent carcinogen. Hypochlorite solutions and hydrochloric acid should therefore be removed from equipment or spaces being decontaminated by formaldehyde.
Formaldehyde is a useful space decontaminant for rooms, cubicles and biological safety cabinets; however, for proper effectiveness, it should only be used when the relative humidity (RH) is between 70% and 90%. Below this range, formaldehyde is less active;
155and, above it, difficult-to-remove polymers are deposited on surfaces. This procedure should only be used by trained personnel.
NOTE: Further information on formaldehyde decontamination is available from Reference 1.21 and, for BSC, AS/NZS 2647 should be consulted.
Glutaraldehyde (1,5-pentanedial) is available as a 2% (w/v) aqueous solution which is activated as a disinfectant by the addition of an alkaline buffer. After activation, its useful life may be restricted to 14 d or 28 d, depending upon the formulation used. It is also available in a stable, glycol-complexed formulation (2% w/v) which does not require activation and which has reduced odour and irritancy. Glutaraldehyde is active against a wide range of microorganisms, including sporing bacteria, although a time period of between 3 h and 10 h (depending upon the manufacturer’s recommendations) is required for reliable sporicidal action. Its main advantages are that it is non-corrosive to metalware and does not harm plastics, rubber or the cement mounting of lenses. Glutaraldehyde is used for the disinfection of certain types of medical equipment. After disinfection, such instruments need to be rinsed well to remove the glutaraldehyde.
Glutaraldehyde is irritating to the eyes and mucous membranes, but less so than is formaldehyde, and may cause dermatitis and respiratory problems in some handlers. The Australian National Exposure Standard, expressed as a TWA, for glutaraldehyde is specified as 0.1 p.p.m. or 0.41 mg/m3. Measures should be taken to protect handlers from exposure to its liquid or vapour. These include the wearing of waterproof, impervious, protective gloves for handling instruments that have been immersed in glutaraldehyde. Containers of glutaraldehyde disinfectant should always be covered and good ventilation, preferably mechanical exhaust ventilation over the container, should be provided. Care should be taken to avoid contamination of the work area by glutaraldehyde solutions.
Peracetic acid (2% v/v) is used as a decontaminant when material is being transferred into plastics isolators containing gnotobiotic animals. It can also be used in disinfectant showers for personnel who are completely covered in waterproof protective clothing. Peracetic acid (2% v/v) is also a good decontaminant for clean, grease-free surfaces.
Peracetic acid solutions have a pungent odour and are irritating to the mucous membranes and highly corrosive. Protective face and respiratory protection should be worn and adequate extractive ventilation provided when the chemical disinfectant is used. A stabilized, non-corrosive formulation has been developed for use in a self-contained system of high-level disinfection of instruments.
The peroxygen system consists of potassium peroxymonosulfate, sodium chloride and an inorganic surfactant acting at a low (acid) pH level. In a 1% w/v concentration, this strongly oxidizing disinfectant is active against a range of microorganisms, including fungi and viruses. However, it has been shown to be ineffective against Mycobacterium spp. and against HIV in the presence of blood (see References 1.22 and 1.23). It is corrosive to metalware and damaging to fabrics but less so than is sodium hypochlorite of equivalent activity.
Hydrogen peroxide is active against a range of microorganisms although fungi are relatively resistant and bacterial spores and enteric viruses require a higher concentration than the 3% w/v generally used for disinfection. A major advantage is the absence of toxic endproducts of decomposition.
156Hydrogen peroxide can also be used as an effective space decontaminant when converted to a vapour (vapourised hydrogen peroxide) in air. A 30% to 35% aqueous solution of liquid hydrogen peroxide is typically flash vapourised and then introduced into the space that is to be decontaminated. The concentration of the vapour in air is typically near 700 ppm, which corresponds to approximately 1.0 mg/litre. Typical exposure time is 120–240 min. As a vapour, hydrogen peroxide competes with water vapour in the air, so humidity may need to be controlled, depending on the equipment used. Standing water should be avoided in the area to be decontaminated. Vapour-phase hydrogen peroxide can be used for the decontamination of containment facilities and primary containment devices such as BSCs. A significant benefit of hydrogen peroxide is that it readily breaks down to oxygen and water. Additional sensing equipment can be a safety requirement due to the fact that hydrogen peroxide vapour at low concentrations is not readily detected visually, by odour or by taste.
Chlorine dioxide is an oxidising agent that can be used as a gaseous decontaminant of containment facilities and BSCs. It is a gas at room temperature and unlike VHP, it does not condense. This enables it to be easily distributed in containment facilities and also facilitates penetration. Like VHP it has the advantage that it is non-carcinogenic and there are no residues resulting from its use. Although it is a gas at normal room temperatures, the relative humidity of the space to be decontaminated is critical for its effectiveness. Decontamination using chlorine dioxide is accomplished at a relative humidity of 65% to 70%, followed by injecting the gas to a typical concentration of 1 mg/litre and exposing the space to be decontaminated for 60–120 min. For further information see Reference 1.28.
A 70% w/w (approximately 80% v/v) solution of ethyl alcohol or a 60–70% v/v solution of isopropyl alcohol provides a useful disinfectant for clean surfaces and the skin. As a skin disinfectant, alcohols are used either alone or in combination with other disinfectants. Emollients, such as glycerol, are also added to counteract the drying effect of alcohols on skin.
Alcohols are active mainly against vegetative bacteria and the lipid-containing viruses and are inactive against spores. However, they are ineffective against Mycobacterium spp. and HIV dried on surfaces in the presence of sputum or serum. Alcohols evaporate from surfaces leaving no residues. However, they may cause swelling of rubber, hardening of plastics and weakening of the cement around lenses in instruments. The alcohols are unsuitable for application to proteinaceous material as they tend to coagulate and precipitate surface proteins which may then result in protection of the microorganisms present. Because of their flammability, alcohol disinfectants should be used sparingly in biological safety cabinets and not with equipment that is likely to produce sparks. In biological safety cabinets, alcohol disinfectants may be used from a dispensing bottle but should not be sprayed.
The synthetic phenolics do not have the pungent odours, highly corrosive and skin irritancy properties of the crude parent compounds, phenol and lysol. They are active against bacteria and lipid-containing viruses but are inactive against spores and the non-lipid-containing viruses. A major advantage of the phenolics is that they are not deactivated by organic matter. They may cause toxic effects if ingested.
Quaternary ammonium compounds(QACs) are cationic detergents with powerful surface active properties. They are effective against Gram-positive bacteria and lipid-containing viruses, e.g. herpes and influenza, but are less active against Gram-negative bacteria and non-lipid-containing viruses and are inactive against Mycobacterium spp. and bacterial
157spores. QACs tend to be inactivated by protein adsorption, anionic soaps and detergents, and cellulosic and synthetic plastics materials. However, they are non-toxic, inexpensive, non-corrosive to metals and non-staining. Because of their detergent properties, they have been used mainly in formulations of cleaning agents in the food industries.
Various formulations of chlorhexidine (as chlorhexidine gluconate) with compatible detergents and ethyl alcohol, or ethyl and isopropyl alcohols, are used as skin disinfectants. The alcoholic formulations have shown to be effective against HIV (see Reference 1.24). In general, aqueous chlorhexidine is active against Gram-positive bacteria, only moderately active against Gram-negative bacteria and inactive against sporing bacteria, Mycobacterium spp. and non-lipid-containing viruses. Alcohols in the skin disinfectant formulations extend the spectrum of activity of chlorhexidine. Chlorhexidine is of low toxicity, except for neurological tissues, and rarely causes hypersensitivity. It is compatible with quaternary ammonium compounds but is incompatible with soap and anionic detergents. Chlorhexidine is widely used in skin disinfectant formulations, but is not recommended as a general disinfectant.
All acids are corrosive and care needs to be taken with their use. Acids are effective against a wide range of microorganisms. Hydrochloric acid solution of 2% concentration can be used in places contaminated with urine, blood, faeces, and in sewage collection areas. Acetic and citric acids are effective for general use against many viruses. A solution of 0.2% citric acid is recommended for personal decontamination. Phosphoric and sulfamic acids are used in food processing areas.
Alkalis have activity against a wide range of microorganisms even in the presence of heavy organic loads in such places as drains and areas contaminated by sewage.
Alkalis are disinfectants of choice for many animal holding areas or animal facilities. 1M sodium hydroxide is a very effective and readily available decontaminant. It retains a high level of activity in the presence of organic matter and is recommended in many situations, such as decontamination of drains and animal houses. Sodium carbonate 4% solution can be used as a wash for animal cages and animal transport vehicles. Sodium metasilicate 5% solution is used as a wash for aircraft and air transport crates.
Ortho-phthalaldehyde (OPA) is an aqueous solution used at 0.55% for high level disinfection of heat sensitive medical instruments. OPA was cleared by the US Food and Drug Administration in October 1999 and has subsequently been frequently used as an alternative to glutaraldehyde, particularly for high level disinfection of flexible endoscopes.
OPA has a rapid anti-mycobacterial effect and is a faster biocidal agent than glutaraldehyde for most common human pathogens. OPA has the potential to cause skin and respiratory sensitivity and therefore, the use of gloves is recommended. Good ventilation in the area of OPA use will assist in reducing respiratory sensitivity.
Medical equipment disinfected with OPA needs to be thoroughly rinsed as there is evidence that residual disinfectant can cause severe allergic reactions. For further information on OPA, see References 1.25 and 1.26.
158Working solutions of disinfectants should be frequently replaced with freshly prepared dilutions from stock solutions. This applies particularly to those disinfectants which are subject to inactivation by organic or other materials, loss of stability or significant dilution through the introduction of wet instruments. Otherwise, the inactivated, exhausted or diluted disinfectants may become contaminated and may even support the growth of the bacterial contaminants. The containers or dispensers used should also be emptied and decontaminated between batches and their contents not merely ‘topped up’.
159| Site or equipment | Routine or preferred method or usage | Acceptable alternative |
|---|---|---|
| Benches and surfaces (not obviously contaminated) | Alcohols e.g. 70% w/w (= 80% v/v) ethyl or 60–70% v/v isopropyl—swabbed (See paragraph F6.9) | Synthetic phenolics* (See paragraph F6.10) |
| Biological safety cabinet (BSC) work surfaces | Alcohols e.g. 70% w/w (= 80% v/v) ethyl—swabbed or high concentration chlorine disinfectant at 5000-10 000 p.p.m. (0.5 –1%) (See paragraph F6.1) or other disinfectant depending on the organism | For BSC with capture hoods, glutaraldehyde† (with cabinet fan operating)—swabbed (see AS/NZS 2647)(See paragraph F 6.4) |
| Room space eg laboratory or animal room, BSC before servicing or testing or after major spill | Formaldehyde vapour (see Paragraph F6.3) | Vaporised hydrogen peroxide (see paragraph F6.7) or Chlorine dioxide (see paragraph F6.8) |
| Centrifuge rotor or sealable bucket after leakage or breakage | Disinfection not the preferred method. Pressure steam sterilizing at 121°C for 15 min recommended (See Clause 10.6) | Glutaraldehyde† for 10 min or synthetic phenolics* for bacterial spills for 10 min(See paragraph F 6.10) |
| Centrifuge bowl after leakage or breakage | Glutaraldehyde† for 10 min (swabbed twice within the 10 min period then wiped with water))(See paragraph F 6.4) | Synthetic phenolics* for bacterial spills for 10 min (See paragraph F 6.10) |
| Discard containers (pipette jars) | Chlorine disinfectant at 2 000–2 500 p.p.m. (0.2–0.25%), | Synthetic phenolics* for bacteriological work (changed weekly) or detergent with pressure steam sterilizing for virus work |
| Equipment surfaces before services or testing | Surfaces disinfected according to manufacturers’ instructions | Alcohol (80% v/v ethyl or 60–70% v/v isopropyl) except when its flammability poses a hazard (See F 6.9)or glutaraldehyde† then water(see F6.4) |
| Gnotobiotic animal isolators | Peracetic acid at 2% v/v —swabbed (see F6.5) | |
| Hand disinfection | Chlorhexidine (0.5–4% w/v) in alcoholic formulations for 2 min (See F6.12) | Isopropyl (60–70% v/v) or ethyl alcohol (80% v/v)) (See F 6.9) with emollients or Povidone-iodine (0.75–1% av I) for 2 min (See F6.2) |
| Hygienic handwash | Chlorhexidine (4% w/v) in detergent formulation (or alcoholic formulations) for 15 s (See F6.12) | Detergent cleansers or soap for 15 s |
| Spills of blood/serum (or viral cultures) | High concentration chlorine at 5000–10 000 p.p.m. (0.5–1%) (See F6.1) for 10 min (active against hepatitis viruses and HIV) | Glutaraldehyde† for 10 min (See F6.4) |
| Spills of bacterial cultures | High concentration chlorine disinfectant at 5 000–10 000 p.p.m. (0.5–1%) or lodophor* for 10 min (See F6.2) | Synthetic phenolics*(unaffected by organic load) for 10 min (See F6.10) 160 |
| Spills of bacterial cultures | High concentration chlorine disinfectant at 5000–10 000 p.p.m. (0.5–1%)* (See F6.1) or lodophor* for 10 min (See F6.2) | Synthetic phenolics* (unaffected by organic load) for 10 min (See F6.10) |
| Animal cages | Wash with detergent followed by pressure steam sterilizing at 121°C for 15 min if infected (See Clause 10.6) | |
| Drains and animal rooms (surfaces) | Sodium hydroxide 1M | |
| * Dilute according to manufacturer’s instructions. † Glutaraldehyde as 2% w/v activated aqueous or 1% w/v glycol-complexed formulations. |
||
| Disinfectant/Decontaminant | Optimum concentration | Minimum Contact time(min)1 | Reference | Bacteria | Fungi | Viruses | Prions | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gram positive | Gram negative | Endospores | Mycobacteria | Enveloped | Non-enveloped | ||||||
| Sterilants | |||||||||||
| Formaldehyde (F6.3) | 8%v/v in 80% ethanol |
10 | 1.21 | + | + | + | + | + | + | + | − |
| Glutaraldehyde (F6.4) | 2% | 10 | 1.30 | + | + | + | + | + | + | + | − |
| Peracetic acid (F6.5) | 2% | 10 | + | + | + | + | + | + | + | − | |
| Vapourised Hydrogen peroxide (F6.7) | 700 ppm =1 mg/l | 120 | 1.31 | + | + | + | + | + | + | + | +2 |
| Chlorine dioxide (F6.8) | 1 mg/l | 60 | + | + | + | + | + | + | + | − | |
| High level disinfectants | |||||||||||
| Peroxygen biocides (F6.6) | 1% | 10 | 1.22, 1.23 | + | + | + | − | + | + | +3 | − |
| Chlorine-releasing compounds (F6.1) | 0.01-5% | 10 | 1.20, 1.16 | + | + | − | +4 | + | + | + | +4 |
| Ortho-phthalaldehyde (F6.14) | 0.55% | 5 | 1.25, 1.26 | + | + | − | + | + | + | + | − 162 |
| Intermediate level disinfectants | |||||||||||
| Iodine/Iodophor (F6.2) | 0.5–2.5% | 10 | + | + | − | − | + | + | − | − | |
| Alcohol (F6.9) | 70-85% | 10 | 1.32 | + | + | − | +5 | − | + | + | − |
| Phenolics (F6.10) | 0.2-3% | 10 | 1.33 | + | + | − | + | + | + | − | +6 |
| Low level disinfectants | |||||||||||
| Quaternary ammonium compounds (F6.11) | 0.1% | 10 | + | + | − | − | − | + | − | − | |
| Chlorhexidine (F6.12) | 2% | 1 | 1.24 | + | + | − | − | − | + | − | − |
| 1 For bacterial endospores, a contact time of up to 720 mins is required for inactivation 2 See Reference 1.31 3 Not in the presence of blood 4 Sodium hypochlorite required a higher concentration of available chlorine (2%) and a contact time of 60 mins to achieve an effective level of decontamination 5 Only effective in the absence of sputum (See Reference 1.32) 6 See Reference 1.33 | |||||||||||
(Informative)
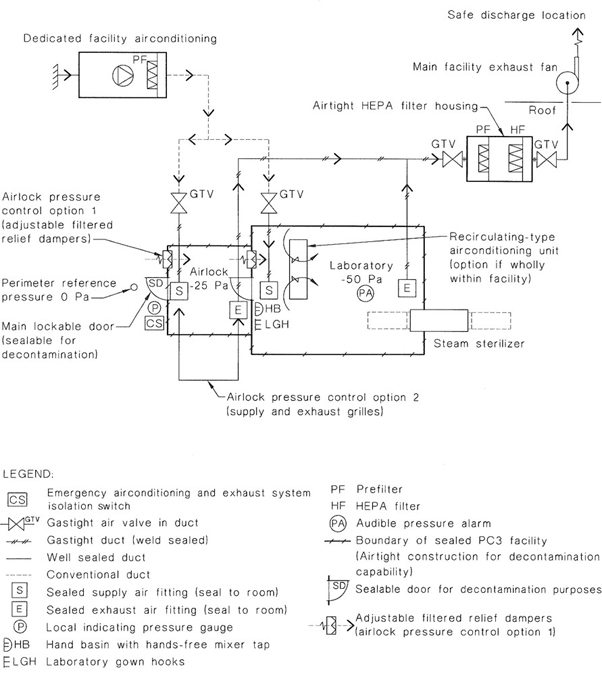
FIGURE G1 SIMPLE PC3 LABORATORY LAYOUT SHOWING DESIGN PRINCIPLES
164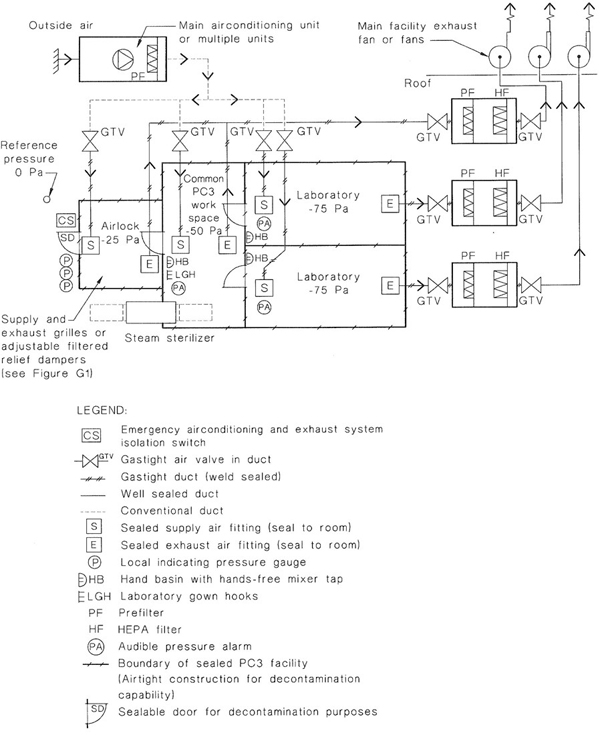
FIGURE G2 ADDITIONAL FEATURES FOR A MULTIPLE ROOM PC3 FACILITY
165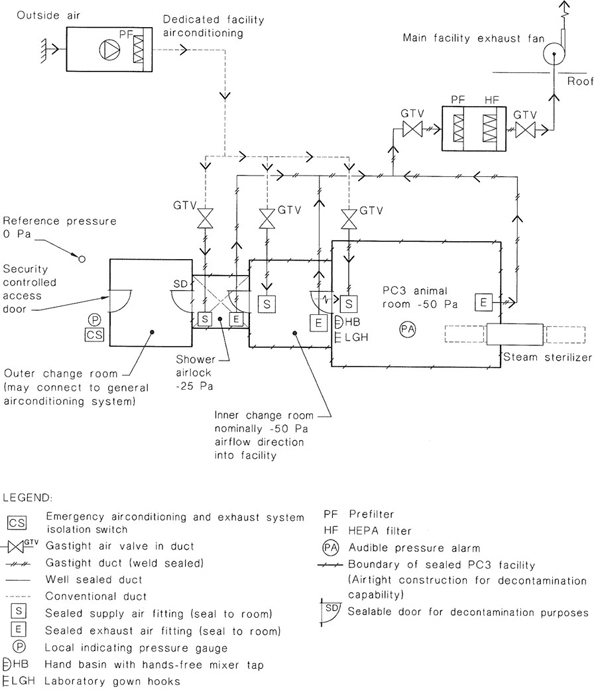
FIGURE G3 PC3 FACILITY WITH SHOWER (appropriate for animal facilities where the room is the primary containment measure)
166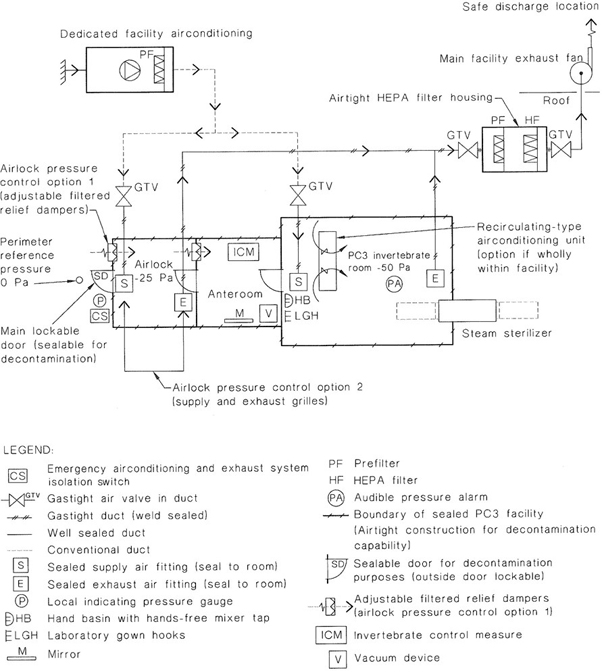
FIGURE G4 PC3 FACILITY WITH ANTEROOM (appropriate for invertebrate facilities)
167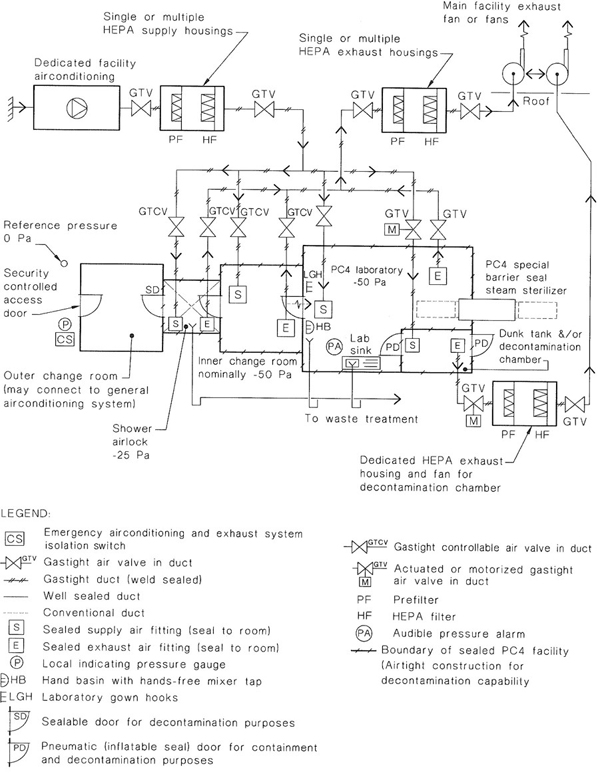
FIGURE G5 PC4 FACILITY LAYOUT SHOWING DESIGN PRINCIPLES
168(Informative)
This Appendix provides recommendations on how to achieve acceptable room airtightness and a method for measuring air leakage.
All PC3 and PC4 facilities have a requirement for containment of aerosols and of gases used in decontamination.
Aerosols generated in the facilities listed in Paragraph H2 are contained using a combination of the following three ways:
Loss of aerosol and gaseous containment can occur due to one or more of the following:
Regular routine testing and maintenance in conjunction with incorporation of appropriate design features can mitigate against loss of containment caused by most of these scenarios. However, there are three issues that influence the degree of structural integrity required of these containment facilities and that form the basis of related risk assessment criteria. These are—
Structural leakage theory for microbiological containment was developed by Graham W. Pickering* for the CSIRO Australian Animal Health Laboratory (AAHL). The theory allows leakage rates of aerosols containing microorganisms or decontamination gas to be simulated. The leakage coefficient β (m3/Pa.s) is used to quantify structural air tightness. Smaller values of β result in more airtight structures. Figure H1 suggests acceptable air leakage values and also identifies the following leakage rates:
The values in Items (b) and (c) refer to leakage rates for high containment animal rooms. The room structure in this instance is the primary containment barrier. These facilities are used to contain exotic disease agents that may have significant political, economic, human health and animal health risk if an outbreak of the exotic disease occurred. This is the reason these facilities are constructed to very stringent leakage criteria.
Many PC3 and PC4 research laboratories do not need to meet the same level of air tightness as they are not dealing with animals and all work is performed in biological safety cabinets that act as the primary containment device within the laboratory structure.
The recommended maximum leakage rate, β, for PC3 and PC4 laboratories is 10−5 at a test pressure of 200 Pa (see Figure H1 and Paragraph H7). This is achievable provided designers and builders pay special attention to joints, penetrations and openings for services.
Metal faced sandwich panel construction or stud wall construction (utilizing two overlapped layers of plasterboard or utilizing two overlapped layers of reinforced cement sheet) can provide a similarly effective well-sealed laboratory finish.
The solution should take into account the requirement for the finished structure to tolerate pressure differentials during normal operation as well as during situations of extreme pressure fluctuation in the event of partial ventilation system failure. This usually requires studs to be positioned at close centres, panelling to be supported at frequent intervals and to be capable of withstanding pressure-generated forces in positive as well as negative directions. The worst case is often immediately after an exhaust fan failure but before the supply fan has been automatically stopped.
*G.W. PICKERING. ANAHL Analysis of Containment Commonwealth of Australia: Department of Transport and Construction, 1982.
170It is recommended that facilities be retested periodically to ensure that the appropriate leakage rate has been maintained during normal use of the laboratory. Facilities should be retested whenever any modifications take place that could affect the integrity of the seal. As an absolute minimum, facilities should be retested every 5 years. It is recommended that consideration be given to test at intervals between 1 year and 5 years. This should be discussed during facility design. The design should incorporate the ability to connect suitable leakage testing equipment.
Effective decontamination with formaldehyde gas has been achieved successfully in practice with a leakage rate of 10−5 at 200 Pa using formaldehyde concentrations of 600–800 p.p.m. (depending on the temperature) and an exposure period of 15 hours. This has been tested for space volumes up to 300 m3. The methodology described in Paragraph F6.3 of Appendix F and Reference 1.21 will normally achieve these concentrations and permits—
Existing laboratories may be capable of achieving successful and safe decontamination with tested leakage rates that exceed the recommended 10−5 at 200 Pa differential pressure. It is not recommended that a laboratory be designed for gaseous decontamination if the leakage rate exceeds 10−4 at 200 Pa differential pressure, without specialist advice.
If gaseous decontamination is proposed within a space that has a leakage coefficient within the range of 10−5 to 10−4 at 200 Pa test pressure, the following additional precautions should be undertaken:
Air leakage can be quantified by using an equilibrium pressure/flow test. This test usually involves the introduction of clean, dry compressed air into the space while monitoring the pressure in the space through a separate pressure tapping. When the pressure is stabilized at the required test pressure (200 Pa or other selected pressure), the inflow of air required to maintain this pressure is measured using a flow meter such as a variable gap meter. The leakage is then recorded in litres per minute.
171Prior to this test, care needs to be taken to ensure that all sources of air or gas pressure within the space are isolated. Doors should be taped with PVC tape and physically restrained to prevent movement under the positive room pressure.
This test can also be performed by extraction of air from the room, thus placing the room under negative pressure. Either of these procedures provides rapid results, freedom from some experimental variable such as the effects of temperature change and requires a low cost test apparatus. All instruments should be appropriately calibrated by an accredited laboratory.
Other test methods involving pressure decay can be adapted to provide a measure of air leakage but have been found less satisfactory.
172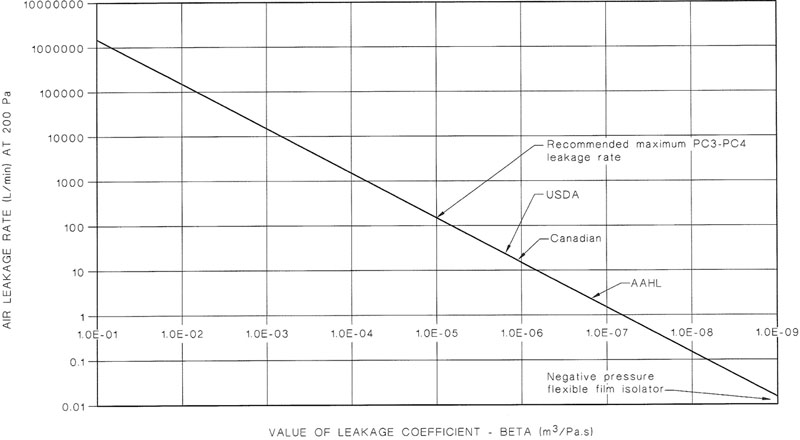
FIGURE H1 LABORATORY AIR LEAKAGE RATES AT 200 Pa DIFFERENTIAL PRESSURE
173NOTE
174NOTE
175NOTE
176Standards Australia
Standards Australia is an independent company, limited by guarantee, which prepares and publishes most of the voluntary technical and commercial standards used in Australia. These standards are developed through an open process of consultation and consensus, in which all interested parties are invited to participate. Through a Memorandum of Understanding with the Commonwealth government, Standards Australia is recognized as Australia’s peak national standards body.
Standards New Zealand
The first national Standards organization was created in New Zealand in 1932. The Standards Council of New Zealand is the national authority responsible for the production of Standards. Standards New Zealand is the trading arm of the Standards Council established under the Standards Act 1988.
Australian/New Zealand Standards
Under a Memorandum of Understanding between Standards Australia and Standards New Zealand, Australian/New Zealand Standards are prepared by committees of experts from industry, governments, consumers and other sectors. The requirements or recommendations contained in published Standards are a consensus of the views of representative interests and also take account of comments received from other sources. They reflect the latest scientific and industry experience. Australian/New Zealand Standards are kept under continuous review after publication and are updated regularly to take account of changing technology.
International Involvement
Standards Australia and Standards New Zealand are responsible for ensuring that the Australian and New Zealand viewpoints are considered in the formulation of international Standards and that the latest international experience is incorporated in national and Joint Standards. This role is vital in assisting local industry to compete in international markets. Both organizations are the national members of ISO (the International Organization for Standardization) and IEC (the International Electrotechnical Commission).
Visit our Web sites
www.standards.org.au
www.standards.co.nz
177